
South East Asia In Vitro Diagnostics Market Size, Share, Trends and Forecast by Test Type, Product, Usability, Application, End Users, and Country, 2025-2033
Market Overview:
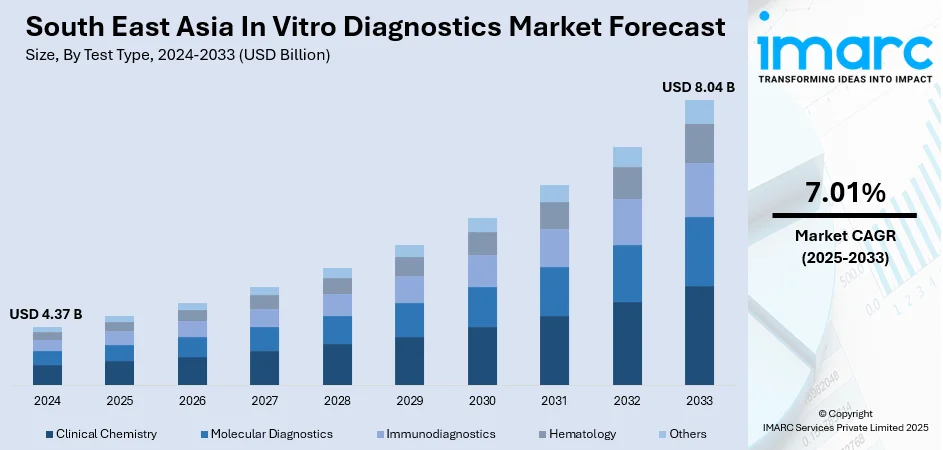
South East Asia in vitro diagnostics market size reached USD 4.37 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 8.04 Billion by 2033, exhibiting a growth rate (CAGR) of 7.01% during 2025-2033. The growing demand for effective diagnostic solutions, burgeoning geriatric population in the region, growing awareness about preventive healthcare, recent technological advancements, and rising healthcare expenditure in South Asian countries represent some of the key factors driving the market.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 4.37 Billion |
| Market Forecast in 2033 | USD 8.04 Billion |
| Market Growth Rate (2025-2033) | 7.01% |
In vitro diagnostics (IVD) refers to medical examinations conducted on samples, such as blood, urine, or tissue, extracted from the human body for detecting diseases, conditions, or infections. It encompasses a wide array of products, including reagents, instruments, and software, which aid in diagnosing and managing various health conditions. The manufacturing process of IVD involves rigorous standards to ensure accuracy, reliability, and safety. IVD exhibits several features, including high sensitivity, specificity, and user-friendliness. It finds applications in numerous areas, such as disease screening, monitoring chronic conditions, therapeutic drug monitoring, infectious disease testing, cancer diagnosis, genetic testing, prenatal testing, and forensic analysis. IVD offers several benefits, such as early disease detection, improved patient outcomes, cost-effectiveness, minimally invasive (MI) testing, rapid results, high accuracy, easy accessibility, enhanced public health surveillance, and support for clinical trials. In addition, IVD aids in guiding treatment decisions, reducing hospital stays, limiting unnecessary treatments, enabling preventive healthcare, supporting effective disease management, assisting in infection control, enhancing laboratory efficiency, contributing to healthcare cost savings, and improving overall patient care.
To get more information on this market, Request Sample
South East Asia In Vitro Diagnostics Market Trends:
The growing demand for effective diagnostic solutions, owing to the heightened prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and cancer is fueling the market growth. Additionally, the burgeoning geriatric population in the region, which is highly prone to various ailments and requires advanced diagnostic services, is contributing to the market growth. Besides this, the growing awareness about preventive healthcare and early disease detection among the population is catalyzing the market growth. Furthermore, recent technological advancements in diagnostic methods that are making diagnostics more accurate and accessible are fueling the market growth. In addition, the rising healthcare expenditure in South East Asia n countries, supporting the development and adoption of advanced IVD technologies, is positively influencing the market growth. Apart from this, the rapid expansion of healthcare infrastructure, including laboratories and diagnostic centers, which creates a conducive environment for the adoption of IVD, is acting as another growth-inducing factor. Moreover, the imposition of governmental support and favorable policies in healthcare are fostering the market growth. Along with this, the increasing medical tourism in the region, attracting a larger patient pool requiring diagnostic services, is driving the market growth. Additionally, the growing trend of personalized medicine, which relies heavily on diagnostic tests, is bolstering the market growth. Furthermore, the rising collaborations and partnerships among key players in the healthcare sector, which contribute to the advancement and distribution of IVD products, are positively impacting the market growth.
South East Asia In Vitro Diagnostics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the regional and country level for 2025-2033. Our report has categorized the market based on test type, product, usability, application, and end users.
Test Type Insights:
- Clinical Chemistry
- Molecular Diagnostics
- Immunodiagnostics
- Hematology
- Others
The report has provided a detailed breakup and analysis of the market based on the test type. This includes clinical chemistry, molecular diagnostics, immunodiagnostics, hematology, and others.
Product Insights:
- Reagents and Kits
- Instruments
A detailed breakup and analysis of the market based on the product have also been provided in the report. This includes reagents and kits and instruments.
Usability Insights:
- Disposable IVD Devices
- Reusable IVD Devices
The report has provided a detailed breakup and analysis of the market based on the usability. This includes disposable IVD devices and reusable IVD devices.
Application Insights:
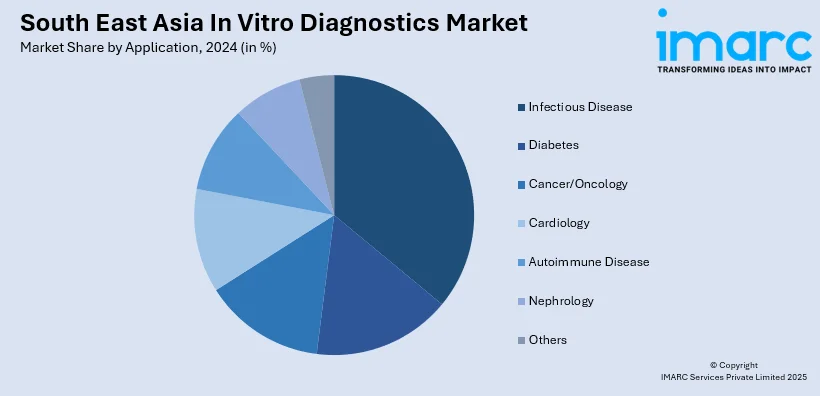
- Infectious Disease
- Diabetes
- Cancer/Oncology
- Cardiology
- Autoimmune Disease
- Nephrology
- Others
A detailed breakup and analysis of the market based on the application have also been provided in the report. This includes infectious disease, diabetes, cancer/oncology, cardiology, autoimmune disease, nephrology, and others.
End Users Insights:
- Hospitals Laboratories
- Clinical Laboratories
- Point-of-Care Testing Centers
- Academic Institutes
- Patients
- Others
The report has provided a detailed breakup and analysis of the market based on the end users. This includes hospitals laboratories, clinical laboratories, point-of-care testing centers, academic institutes, patients, and others.
Country Insights:
- Indonesia
- Thailand
- Singapore
- Philippines
- Vietnam
- Malaysia
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Indonesia, Thailand, Singapore, Philippines, Vietnam, Malaysia, and Others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape in the market. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South East Asia In Vitro Diagnostics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Test Types Covered | Clinical Chemistry, Molecular Diagnostics, Immunodiagnostics, Hematology, Others |
| Products Covered | Reagents and Kits, Instruments |
| Usabilities Covered | Disposable IVD Devices, Reusable IVD Devices |
| Applications Covered | Infectious Disease, Diabetes, Cancer/Oncology, Cardiology, Autoimmune Disease, Nephrology, Others |
| End Users Covered | Hospitals Laboratories, Clinical Laboratories, Point-of-Care Testing Centers, Academic Institutes, Patients, Others |
| Countries Covered | Indonesia, Thailand, Singapore, Philippines, Vietnam, Malaysia, Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South East Asia in vitro diagnostics market performed so far and how will it perform in the coming years?
- What has been the impact of COVID-19 on the South East Asia in vitro diagnostics market?
- What is the breakup of the South East Asia in vitro diagnostics market on the basis of test type?
- What is the breakup of the South East Asia in vitro diagnostics market on the basis of product?
- What is the breakup of the South East Asia in vitro diagnostics market on the basis of usability?
- What is the breakup of the South East Asia in vitro diagnostics market on the basis of application?
- What is the breakup of the South East Asia in vitro diagnostics market on the basis of end users?
- What are the various stages in the value chain of the South East Asia in vitro diagnostics market?
- What are the key driving factors and challenges in the South East Asia in vitro diagnostics?
- What is the structure of the South East Asia in vitro diagnostics market and who are the key players?
- What is the degree of competition in the South East Asia in vitro diagnostics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South East Asia in vitro diagnostics market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South East Asia in vitro diagnostics market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South East Asia in vitro diagnostics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












