Acute Bacterial Skin and Skin Structure Infections Market Size to Reach USD 14.50 Billion by 2035, Impelled by Advancements in Early Detection
Acute Bacterial Skin and Skin Structure Infections Market Outlook 2025-2035:
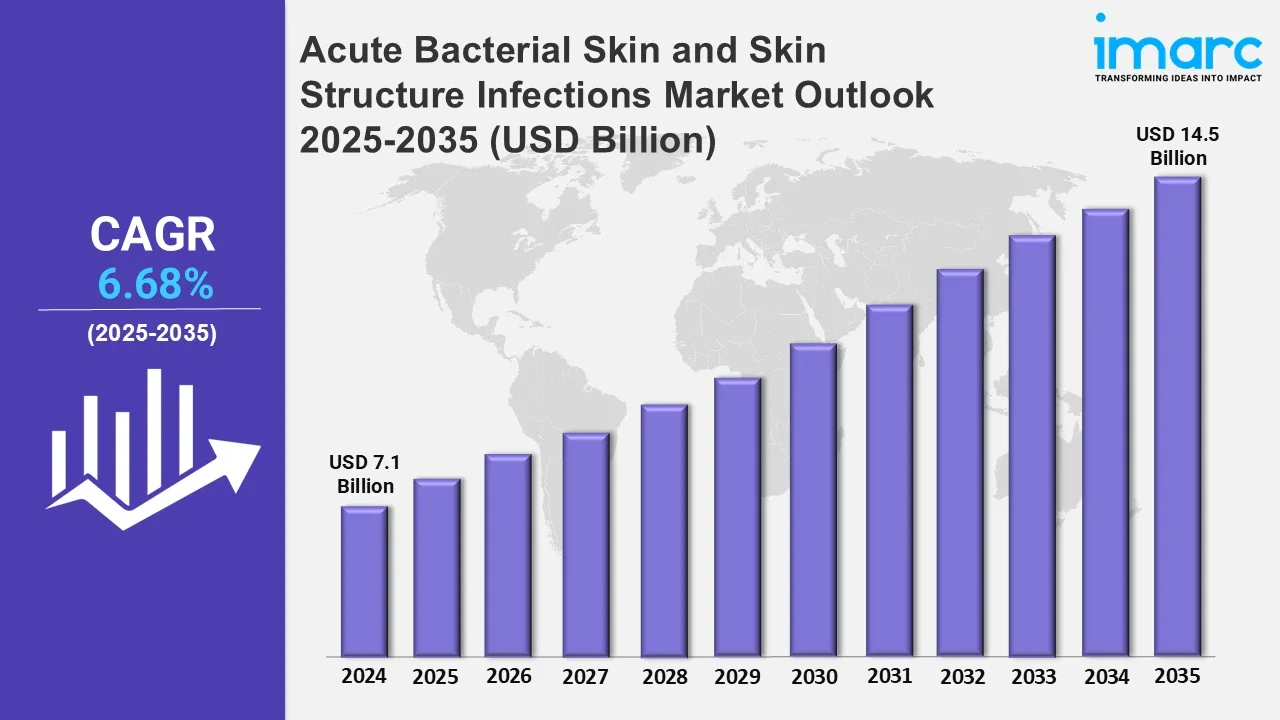
The 7 major acute bacterial skin and skin structure infections market reached a value of USD 7.10 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 14.50 Billion by 2035, exhibiting a growth rate (CAGR) of 6.68% during 2025-2035. The market is driven by the emerging popularity of targeted therapies, which are designed to specifically aim at certain molecules or proteins involved in the disease process, thereby ensuring more precise and effective treatment. Additionally, the development of innovative treatments is further propelling the market growth.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Acute Bacterial Skin and Skin Structure Infections Market
The market for acute bacterial skin and skin structure infections (ABSSSI) is witnessing significant growth, driven by advancements in early detection and diagnostic technologies. Recent technological innovations in microbiological and molecular diagnostics are playing a crucial role in improving patient outcomes and shaping market trends. One of the most transformative developments is the use of rapid molecular diagnostics, such as polymerase chain reaction (PCR) and next-generation sequencing (NGS), which allow for the precise identification of bacterial pathogens in a matter of hours rather than days. These techniques help in the early differentiation between bacterial and non-bacterial infections, enabling clinicians to administer targeted antibiotic therapy and minimize unnecessary antibiotic use, thereby reducing antimicrobial resistance. Point-of-care (POC) testing has also emerged as a game-changer in ABSSSI diagnostics. These portable, easy-to-use devices provide real-time results, improving clinical decision-making and reducing hospital stays. Furthermore, artificial intelligence (AI)-driven diagnostic tools and automated imaging technologies are enhancing the accuracy of infection detection, supporting clinicians in diagnosing ABSSSI with greater efficiency. The growing adoption of biomarker-based diagnostics is another key advancement. Biomarkers like procalcitonin (PCT) and C-reactive protein (CRP) are increasingly being used to assess infection severity and guide treatment strategies. These developments not only improve patient care but also contribute to the expansion of the ABSSSI market by driving demand for innovative diagnostic solutions.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The acute bacterial skin and skin structure infections market is witnessing substantial expansion, driven by the development of novel therapies and pharmacological treatments. With the rising incidence of resistant bacterial strains, there is an increasing need for innovative antimicrobial agents that offer improved efficacy, reduced side effects, and shorter treatment durations. Pharmaceutical companies and research institutions are actively working on advanced treatment options to address these challenges, thereby fueling market growth. One of the key drivers of this expansion is the introduction of next-generation antibiotics, including novel lipoglycopeptides, oxazolidinones, and tetracycline derivatives, which provide enhanced potency against drug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA). These new antibiotics, such as dalbavancin, oritavancin, and tedizolid, offer advantages like extended half-lives, allowing for fewer doses and better patient compliance. In addition to traditional antibiotics, the market is also seeing growth in non-antibiotic treatments, such as bacteriophage therapy, antimicrobial peptides, and monoclonal antibodies, which offer alternative mechanisms to combat resistant infections. These innovative approaches help reduce reliance on conventional antibiotics and mitigate the growing threat of antimicrobial resistance. Moreover, advancements in drug delivery systems, such as long-acting injectable formulations and nanotechnology-based delivery, are improving the pharmacokinetics and therapeutic efficacy of ABSSSI treatments. These innovations enable sustained drug release, reducing dosing frequency and enhancing patient outcomes.
Marketed Therapies in Acute Bacterial Skin and Skin Structure Infections Market
Orbactiv (Oritavancin): Melinta Therapeutics
Orbactiv (Oritavancin) is an antibacterial medication that treats acute bacterial skin and skin structure infections caused by susceptible Gram-positive bacteria. Oritavancin inhibits susceptible gram-positive organisms via three distinct methods. First, it binds to the stem peptide of peptidoglycan precursors, preventing transglycosylation (polymerization). This process often happens during cell wall formation. Second, oritavancin inhibits crosslinking during bacterial cell wall manufacturing by binding to the cell wall's pentaglycyl peptide bridging segments. Finally, this medicine functions by disrupting the bacterial cell membrane and interfering with its integrity, resulting in cell death via a variety of processes.
Nuzyra (Omadacycline): Paratek Pharmaceuticals
Nuzyra (Omadacycline) is indicated for the treatment of acute bacterial skin and skin structure infections caused by omadacycline-susceptible organisms in adults. Omadacycline can be bacteriostatic or bacteriocidal, depending on the organism involved. It inhibits bacterial protein synthesis while not affecting DNA, RNA, or peptidoglycan production. Omadacycline binds to the main tetracycline binding site on the bacterial 30s ribosomal subunit with high selectivity. There, it blocks protein synthesis, affecting many aspects of cellular function and resulting in either cell death or stasis.
Dalvance (Dalbavancin): Allergan
Dalvance (Dalbavancin) is an antibacterial medication used to treat acute bacterial skin and skin structure infections caused by susceptible Gram-positive bacteria strains. Dalbavancin's bactericidal activity is principally due to its suppression of cell wall production. Dalbavancin specifically inhibits the incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits into the peptidoglycan matrix, which is the primary structural component of Gram-positive cell walls. The large hydrophilic molecule can create hydrogen bond contacts with the terminal D-alanyl-D-alanine moieties of NAM/NAG-peptides, which is often a five-point interaction. Dalbavancin's binding to the D-Ala-D-Ala site prevents the NAM/NAG-peptide subunits from being incorporated into the peptidoglycan matrix. Furthermore, dalbavancin affects bacterial-cell membrane permeability and RNA production.
Sivextro (Tedizolid): Merck & Co
Sivextro (Tedizolid) is an antibiotic of the oxazolidinone class that is used to treat acute bacterial skin and skin structure infections caused by susceptible isolates of Gram-positive microorganisms in adults and pediatric patients aged 12 and above. In vivo, plasma phosphatases convert tedizolid phosphate into microbiologically active tedizolid. It interacts with the bacterial 23S ribosome initiation complex and binds to the 50S subunit, preventing the development of the 70S complex. As a result, tedizolid prevents bacterial translation and protein production.
Leading Companies in the Acute Bacterial Skin and Skin Structure Infections Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global acute bacterial skin and skin structure infections market, several leading companies are at the forefront of developing integrated platforms to enhance the management of acute bacterial skin and skin structure infections. Some of the major players include Melinta Therapeutics, Paratek Pharmaceuticals, and Merck & Co. These companies are driving innovation in the acute bacterial skin and skin structure infections market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In August 2024, Sun Pharmaceutical Industries announced the launch of Tedizolid Phosphate tablets 200 mg in India under the brand name STARIZO. STARIZO (Tedizolid Phosphate) is a new oxazolidinone-class antibiotic that treats acute bacterial skin and skin structure infections. Sun Pharma had acquired rights from Merck Sharp & Dohme Singapore Trading to develop, manufacture, and market Tedizolid Phosphate in India.
Key Players in Acute Bacterial Skin and Skin Structure Infections Market:
The key players in the Acute Bacterial Skin and Skin Structure Infections market who are in different phases of developing different therapies are Melinta Therapeutics, Paratek Pharmaceuticals, Allergan, Merck & Co, and Others.
.webp)
Regional Analysis:
The major markets for acute bacterial skin and skin structure infections include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for acute bacterial skin and skin structure infections while also representing the biggest market for its treatment. This can be attributed to the growing incidence of infections caused by methicillin-resistant Staphylococcus aureus and other resistant pathogens that have heightened the demand for novel and effective antimicrobial treatments.
Moreover, the expanding aging population, which is more susceptible to ABSSSI due to weakened immune systems and comorbidities such as diabetes and chronic wounds, is also bolstering the market growth. Additionally, an increasing number of hospitalizations and surgical procedures in the U.S. contribute to a higher risk of skin and soft tissue infections, necessitating rapid and effective treatments.
Besides this, regulatory support and financial incentives from organizations such as the U.S. FDA are also fueling market growth. The FDA’s Generating Antibiotic Incentives Now (GAIN) Act and fast-track approvals for novel antimicrobial drugs encourage pharmaceutical companies to invest in ABSSSI treatment innovations.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the acute bacterial skin and skin structure infections market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the acute bacterial skin and skin structure infections market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current acute bacterial skin and skin structure infections marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












