Colorectal Cancer Market Size to Reach USD 17.9 Billion by 2035, Impelled by Advancements in Early Diagnosis & Screening
Colorectal Cancer Market Outlook 2025-2035:
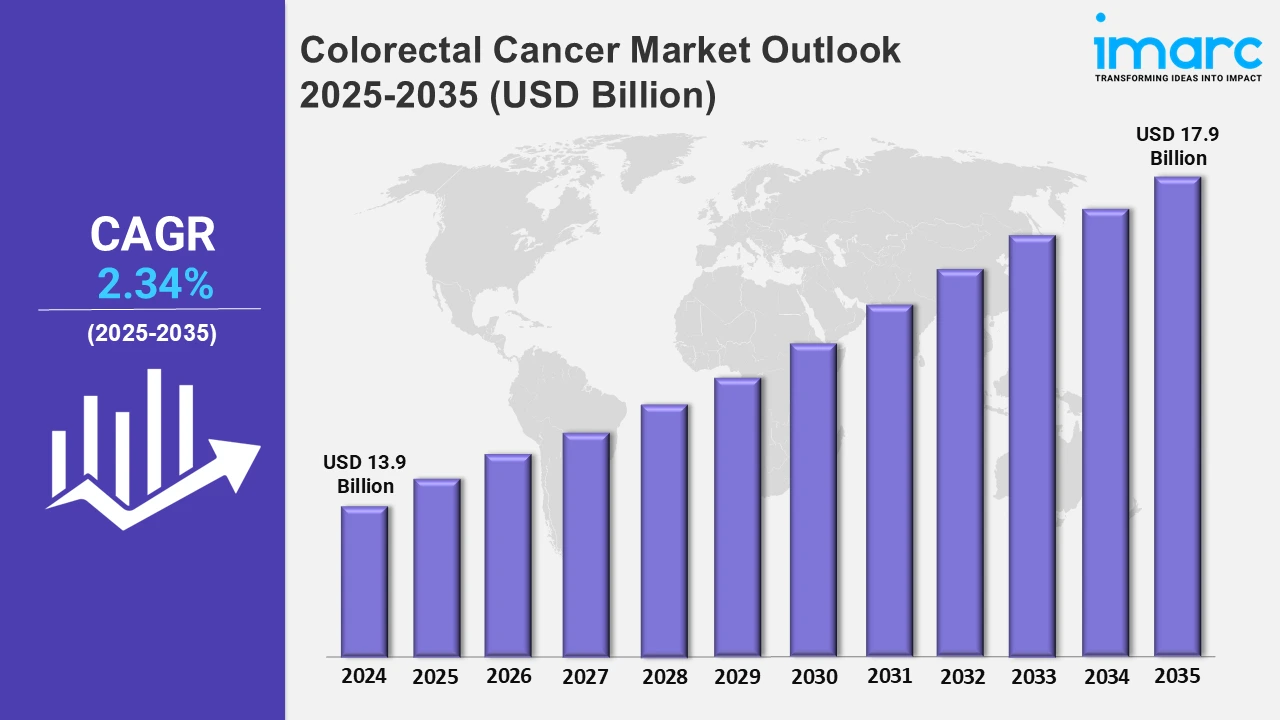
The Colorectal cancer market reached a value of USD 13.9 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 17.9 Billion by 2035, exhibiting a growth rate (CAGR) of 2.34% during 2025-2035. The colorectal cancer (CRC) treatment market is undergoing a dynamic transformation, driven by advancements in diagnostics and therapeutic innovations. Early and accurate diagnosis is becoming increasingly vital, leveraging liquid biopsy, next-generation sequencing (NGS), AI-powered imaging, and biomarker-based screening tests. These cutting-edge technologies enable healthcare professionals to detect CRC at earlier stages, differentiate between genetic subtypes, and personalize treatment approaches, ultimately improving patient survival rates. Simultaneously, the therapeutic landscape is evolving with the introduction of targeted therapies, immunotherapies, and novel drug combinations. While traditional treatments such as surgery, chemotherapy, and radiation therapy remain essential, immune checkpoint inhibitors, monoclonal antibodies, and tumor microenvironment-targeting agents are revolutionizing disease management with enhanced efficacy and fewer side effects. Additionally, combination therapies, including immunotherapy with chemotherapy or targeted agents, are improving treatment response rates and long-term disease control. These advancements are reshaping colorectal cancer care, offering new hope for patients and improving overall treatment outcomes worldwide.
To get more information on this market, Request Sample
Expansion of Targeted & Immunotherapies in Colorectal Cancer Treatment
The colorectal cancer treatment landscape is witnessing significant advancements with the increased development and approval of targeted therapies and immunotherapies. Immune checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4 inhibitors, are improving survival outcomes, particularly in microsatellite instability-high (MSI-H) and mismatch repair-deficient (dMMR) CRC patients. Monoclonal antibodies targeting EGFR (e.g., Cetuximab, Panitumumab) and VEGF (e.g., Bevacizumab) are enhancing precision treatment by inhibiting tumor growth and angiogenesis. The rise of personalized medicine, driven by biomarker-based patient selection, is optimizing therapy effectiveness while minimizing side effects. Combination strategies, such as immunotherapy with chemotherapy or targeted agents, are further enhancing treatment response rates. Ongoing clinical trials continue to explore novel agents and next-generation immune-based approaches, offering hope for improved disease control and long-term survival.
Rising Investments in Oncology R&D Driving Colorectal Cancer Advancements
The colorectal cancer (CRC) market is benefiting from significant investments in oncology research and development (R&D), leading to breakthrough innovations in treatment strategies. Increased funding is accelerating the discovery of novel drug candidates, including next-generation immunotherapies, targeted therapies, and tumor microenvironment modulators. Combination therapies, such as immune checkpoint inhibitors with chemotherapy or targeted agents, are being extensively studied to enhance treatment efficacy. Precision medicine approaches, leveraging biomarkers and genetic profiling, are improving patient selection for tailored therapies, reducing side effects and increasing survival rates. Public and private sector investments are supporting large-scale clinical trials to bring promising therapies to market faster. Collaborations between pharmaceutical companies, biotech firms, and academic institutions are driving drug innovation and regulatory approvals. These investments are reshaping colorectal cancer treatment, offering new hope for patients with advanced or treatment-resistant disease.
Marketed Therapies in the Colorectal Cancer Market
Vectibix (Panitumumab) – Amgen
Vectibix (Panitumumab) is a monoclonal antibody used in the treatment of metastatic colorectal cancer (mCRC). It specifically targets the epidermal growth factor receptor (EGFR) to inhibit cancer cell growth in RAS wild-type CRC patients.
Avastin (Bevacizumab) - Roche
Avastin (Bevacizumab) – Genentech/Roche is an anti-VEGF monoclonal antibody used in the treatment of metastatic colorectal cancer (mCRC). It works by inhibiting vascular endothelial growth factor (VEGF), preventing tumor angiogenesis and restricting blood supply to cancer cells.
Stivarga (Regorafenib) - Bayer Healthcare
Stivarga (Regorafenib) is an oral multi-kinase inhibitor approved for the treatment of metastatic colorectal cancer (mCRC) in patients who have progressed after standard therapies. It targets multiple pathways, including angiogenesis, oncogenesis, and tumor microenvironment regulation, to slow cancer progression.
Erbitux (Cetuximab) - Eli Lilly and Company
Erbitux (Cetuximab) – Eli Lilly and Company is a monoclonal antibody used for the treatment of metastatic colorectal cancer (mCRC) in RAS wild-type patients. It specifically targets the epidermal growth factor receptor (EGFR) to inhibit tumor growth and enhance the effects of chemotherapy.
Emerging Therapies in the Colorectal Cancer Market
XL 092 – Exelixis
Exelixis is advancing (XL092), an investigational tyrosine kinase inhibitor, in combination with atezolizumab for the treatment of metastatic colorectal cancer (mCRC). The STELLAR-303 Phase 3 trial is evaluating this combination against regorafenib in patients with microsatellite stable or low microsatellite instability mCRC who have progressed after standard therapies. This study aims to address the limited treatment options and poor prognosis associated with advanced mCRC.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| XL 092 | Exelixis | Axl receptor tyrosine kinase inhibitors; Proto-oncogene protein c-mer inhibitors; Proto-oncogene protein c-met inhibitors; Vascular endothelial growth factor receptor antagonists | Oral |
Detailed list of emerging therapies for Colorectal Cancer provided in the final report…
Leading Companies in the Colorectal Cancer Market:
The colorectal cancer (CRC) treatment market is undergoing a significant transformation, driven by competition and breakthrough innovations from leading pharmaceutical and biotech companies. Key players such as Amgen, Roche, Bayer Healthcare, Eli Lilly, and Exelixis are heavily investing in targeted therapies, immunotherapies, and combination treatments to improve CRC management. A major shift is occurring, moving beyond traditional chemotherapy and radiation therapy toward precision medicine approaches that specifically target tumor growth, angiogenesis, and immune evasion mechanisms. This is evident in the development of novel therapies such as Vectibix (Panitumumab), Avastin (Bevacizumab), Stivarga (Regorafenib), and Erbitux (Cetuximab), which aim to enhance treatment efficacy, reduce side effects, and improve survival outcomes. Supported by ongoing clinical trials, biomarker-driven therapies, and regulatory advancements, the CRC treatment landscape is evolving toward personalized and more effective treatments, offering new hope for patients and better long-term disease management.
Key Players in the Colorectal Cancer Market:
The key players in the Colorectal Cancer market who are in different phases of developing different therapies are Amgen, Roche, Bayer Healthcare, Eli Lilly, Exelixis, and others.

Regional Analysis:
The colorectal cancer (CRC) treatment market is heavily concentrated in developed regions such as the United States, Germany, the UK, France, Italy, Spain, and Japan, where advancements in oncology research and precision medicine are driving innovation. The United States plays a pivotal role in this market due to its leadership in pharmaceutical R&D, biomarker-driven therapies, and immuno-oncology advancements. While current treatment approaches focus on chemotherapy, radiation therapy, and surgical interventions, significant progress is being made in understanding the molecular and genetic landscape of CRC, leading to the development of targeted therapies and immunotherapies. The market is also being fueled by increased investments in oncology research, accelerated regulatory approvals for novel treatments, and strategic collaborations between pharmaceutical companies, academic institutions, and healthcare providers. These efforts are expanding treatment options, improving disease management, and ultimately enhancing survival outcomes and quality of life for patients affected by colorectal cancer.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the colorectal cancer market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the colorectal cancer market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current colorectal cancer-marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












