Epilepsy Market Size to Reach USD 2.70 Billion by 2035, Impelled by Advancements in Personalized Medicine
Epilepsy Market Outlook 2025-2035:
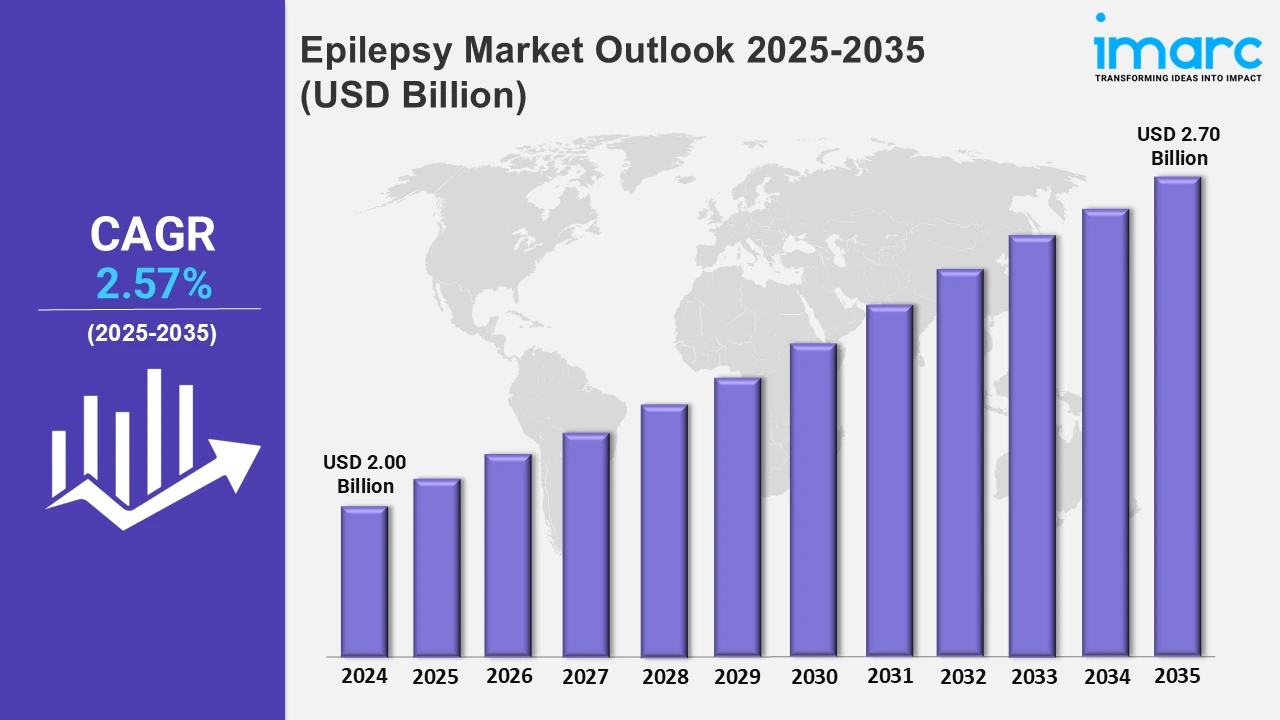
The 7 major epilepsy market reached a value of USD 2.00 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2.70 Billion by 2035, exhibiting a growth rate (CAGR) of 2.57% during 2025-2035. The market is driven by the ongoing research into extended-release formulations encouraging longer dosing periods with progressively less variability in the serum concentrations of the drugs increasing efficacy beyond immediate-release formulations. Also, the advancement of targeted therapy or new therapeutics fuels market growth.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Epilepsy Market
The market for epilepsy is expanding rapidly as a result of emerging technologies for early detection and diagnosis, which are pivotal in bettering the management of diseases and outcomes for patients. Further advancements in high-resolution electroencephalography (EEG) stand out as among the most important advancements in the diagnosis of epilepsy. Modern EEG systems include wireless and wearable devices that provide real-time monitoring of brain activity, which enables continuous detection of seizures away from hospital environments. Advanced signal processing and AI algorithms further enhance the accuracy of EEG, facilitating the differentiation of seizures due to epilepsy, from other seizure-like conditions. Neuroradiology technologies like high-definition magnetic resonance imaging (MRI) and positron emission tomography (PET) are also improving the localization of the seizure activity within the brain. The imaging techniques aid in recognizing structural abnormalities within the brain lesions, tumors, or malformations that may contribute to epilepsy, thus allowing improved planning for treatment. Research on biomarkers is leading to the development of blood-based and genetic tests that can predict the onset and severity of epilepsy. Liquid biopsy methodologies and gene sequencing will now facilitate the early identification of subtypes in epilepsy, thereby allowing targeted therapy selections for patients. Another revolution in the field of epilepsy diagnostics is the induction of AI and machine learning in epilepsy diagnosis. AI-powered systems can analyze huge data from patients to identify subtle seizure patterns, predict seizures, and automatically provide diagnostic insights. Advancements in technology for these purposes will still keep making epilepsy diagnosis less complicated and more accessible, which shall drive the growth of the market. Within such a context, early and accurate diagnosis will allow timely treatment, with reduced complications associated with seizures, and would thus improve the quality of life for patients with epilepsy.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The epilepsy market is expanding significantly due to the development of novel therapies and pharmacological treatments that offer improved seizure control, fewer side effects, and personalized treatment options. One of the key advancements in epilepsy treatment is the introduction of next-generation antiepileptic drugs (AEDs) that target specific neurotransmitter pathways with greater efficacy and reduced side effects. Drugs such as cenobamate and brivaracetam have demonstrated superior seizure control in drug-resistant epilepsy patients. Additionally, selective sodium channel blockers and GABA receptor modulators are improving treatment outcomes while minimizing cognitive and behavioral side effects. Another major breakthrough is the development of gene therapies aimed at treating genetic forms of epilepsy. Advances in CRISPR and antisense oligonucleotide (ASO) therapies are enabling precise genetic modifications to correct underlying mutations that cause epilepsy, paving the way for potential curative treatments. Neuromodulation therapies, such as vagus nerve stimulation (VNS), responsive neurostimulation (RNS), and deep brain stimulation (DBS), are expanding treatment options for drug-resistant epilepsy patients. These implantable devices regulate abnormal brain activity, reducing seizure frequency and improving quality of life. The rise of personalized medicine and biomarker-driven treatment approaches is also transforming epilepsy care by tailoring therapies to an individual’s genetic and metabolic profile. With continued research, regulatory approvals, and increasing investment in innovative treatments, the epilepsy market is poised for sustained growth. These novel therapies are addressing unmet needs, improving patient outcomes, and significantly enhancing epilepsy management worldwide.
Marketed Therapies in Epilepsy Market
Keppra (Levetiracetam): GlaxoSmithKline/Otsuka Pharmaceuticals/UCB
Keppra (levetiracetam) is a novel anticonvulsant that is used as an adjunct medicine to treat partial onset, myoclonic, and generalized tonic-clonic seizures in epilepsy patients. It works by attaching to a protein on the surface of brain cells known as synaptic vesicle protein 2A (SV2A), which regulates neurotransmitter release, thereby lowering aberrant neuronal firing and preventing seizures. Essentially, it works by preventing excessive neurotransmitter release at the synapse, which is considered to have a distinct mode of action when compared to other antiepileptic medications.
Topamax (Topiramate): Janssen
Topamax (Topiramate) is an antiepileptic medicine used in the control of seizures in epilepsy and in the prevention and treatment of migraine. It augments GABA-A receptor activity in the brain at non-benzodiazepine receptor sites and diminishes glutamate action at both AMPA and kainate receptors. GABA-A receptors naturally inhibit neuronal activity, whereas glutamate receptors activate it. This property of Topiramate to enhance GABAA while also decreasing glutamate activity decreases neuronal excitability and prevents seizure activity and migraine. Exacerbation of seizure activity is diminished by inhibition of the voltage-gated sodium channels by Topiramate. Its weak inhibition of carbonic anhydrase may also play a role in its antiepileptic efficacy.
Zonegran (Zonisamide): Eisai Co Ltd
Zonegran (Zonisamide) is a sulfonamide anticonvulsant that is used as a supplementary treatment for individuals who have partial-onset seizures. There are sodium and calcium channel actions upon which one can attribute the antiepileptic activities of the drug. Zonisamide, acting on sodium channels with a resultant drop in voltage-dependent transient inward currents, can stabilize neuronal membranes, thereby diminishing hypersynchronization. It alters T-type calcium currents while showing no effective change on L-type calcium currents. Zonisamide interferes with synaptically driven electrical activity by modulating the synthesis, release, and degradation of many neurotransmitters, such as glutamate, GABA, dopamine, serotonin (5-hydroxytryptamine 5-HT), and acetylcholine. Besides, it also binds to the GABA/benzodiazepine receptor ionophore complex but does not alter chloride flux.
Emerging Therapies in Epilepsy Market
YKP3089: SK biopharmaceuticals
YKP3089, also known as Cenobamate, is an antiepileptic medication created by SK Pharmaceuticals that is used to treat partial-onset seizures in adult patients. YKP3089 is considered to operate by two distinct mechanisms: one through enhancing inhibitory currents by positive modulation of GABA-A receptors while another through the inhibition of persistent sodium current, decreasing excitatory currents. Inhibition of voltage-gated sodium channels raises the threshold for action-potential generation while decreasing the number of action potentials.
Ganaxolone: Marinus Pharmaceuticals
The cyclin-dependent kinase-like 5 deficiency disorder is treated with Ganaxolone medication, which treats the seizures. Ganaxolone is expected to antagonize overly stimulated neurons by regulating synaptic as well as extrasynaptic GABAA receptors. Extrasynaptic receptor stimulation might be another stabilizing mechanism for Ganaxolone, which may differentiate it from other drugs that act to increase GABA signaling. It binds to the GABAA receptor at allosteric sites, thereafter, modulating and opening the chloride ion channel, hence hyperpolarizing the cell. This hyperpolarization inhibits neurotransmission and therefore reduces the probability of a successful action potential (depolarization).
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| YKP3089 | SK biopharmaceuticals | GABA A receptor modulators; Sodium channel antagonists | Oral |
| Ganaxolone | Marinus Pharmaceuticals | GABA A receptor modulators | Intravenous |
Detailed list of emerging therapies in Epilepsy is provided in the final report…
Leading Companies in the Epilepsy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global epilepsy market, several leading companies are at the forefront of developing integrated platforms to enhance the management of epilepsy. Some of the major players include GlaxoSmithKline, Otsuka Pharmaceuticals, and UCB. These companies are driving innovation in the epilepsy market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In July 2024, Glenmark Pharmaceuticals Ltd announced that it received final approval from the U.S. health regulator for its generic Topiramate capsules, which are used to treat specific types of seizures. The approved medicine is bioequivalent and therapeutically equivalent to Janssen Pharmaceuticals' Topamax capsules, and it will be supplied in the United States by Glenmark Therapeutics Inc.
In June 2024, UCB announced that the Japanese Ministry of Health, Labor, and Welfare (MHLW) has authorized BRIVIACT (brivaracetam) to be used as a monotherapy and adjunctive therapy for the treatment of partial-onset seizures in adults suffering from epilepsy, with or without secondary generalization. Without escalation to a therapeutic dose, brivaracetam is initiated on its first day of administration.
Key Players in Epilepsy Market:
The key players in the Epilepsy market who are in different phases of developing different therapies are SK biopharmaceuticals, Marinus Pharmaceuticals, GlaxoSmithKline, Otsuka Pharmaceuticals, UCB, Janssen, Eisai Co Ltd, Supernus Pharmaceuticals, Cerevel Therapeutics, Biohaven Pharmaceuticals, and Others.

Regional Analysis:
The major markets for epilepsy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for epilepsy while also representing the biggest market for its treatment. This can be attributed to the growing number of drug-resistant epilepsy cases, where patients fail to respond to traditional antiepileptic drugs, which is fueling the need for novel therapies.
Moreover, the rise in neuromodulation technologies, such as vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation, is also contributing to market growth. These devices provide effective alternatives for patients with drug-resistant epilepsy, offering long-term seizure control and better quality of life.
Besides this, next-generation antiepileptic drugs, including cenobamate and brivaracetam, offer improved efficacy and safety profiles, making them viable options for patients with uncontrolled seizures. Additionally, gene and cell therapies are emerging as potential curative treatments, particularly for genetic forms of epilepsy, driving further market expansion.
Recent Developments in Epilepsy Market:
- In December 2024, SK Biopharmaceuticals reported favorable findings from a Phase 3 clinical trial of cenobamate (YKP3089) in patients with epilepsy in Asia. The trial confirmed the drug's safety and efficacy in treating uncontrolled seizures. Based on these results, the Asian partners of cenobamate intend to submit New Drug Applications (NDAs) in their respective nations.
- In May 2024, Supernus Pharmaceuticals, Inc. provided results of the planned interim analysis of the exploratory open-label Phase 2a clinical study of SPN-817 for treatment-resistant seizures. This research assesses the safety and tolerability of SPN-817 for adult patients with treatment-resistant seizures. It also finds effective doses of the product in different types of treatment-resistant seizures.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the epilepsy market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the epilepsy market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current epilepsy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.





.webp)




.webp)












