Esophageal Cancer Market Size to Reach USD 17.6 Billion by 2035, Impelled by Advancements in Early Detection
Esophageal Cancer Market Outlook 2025-2035:
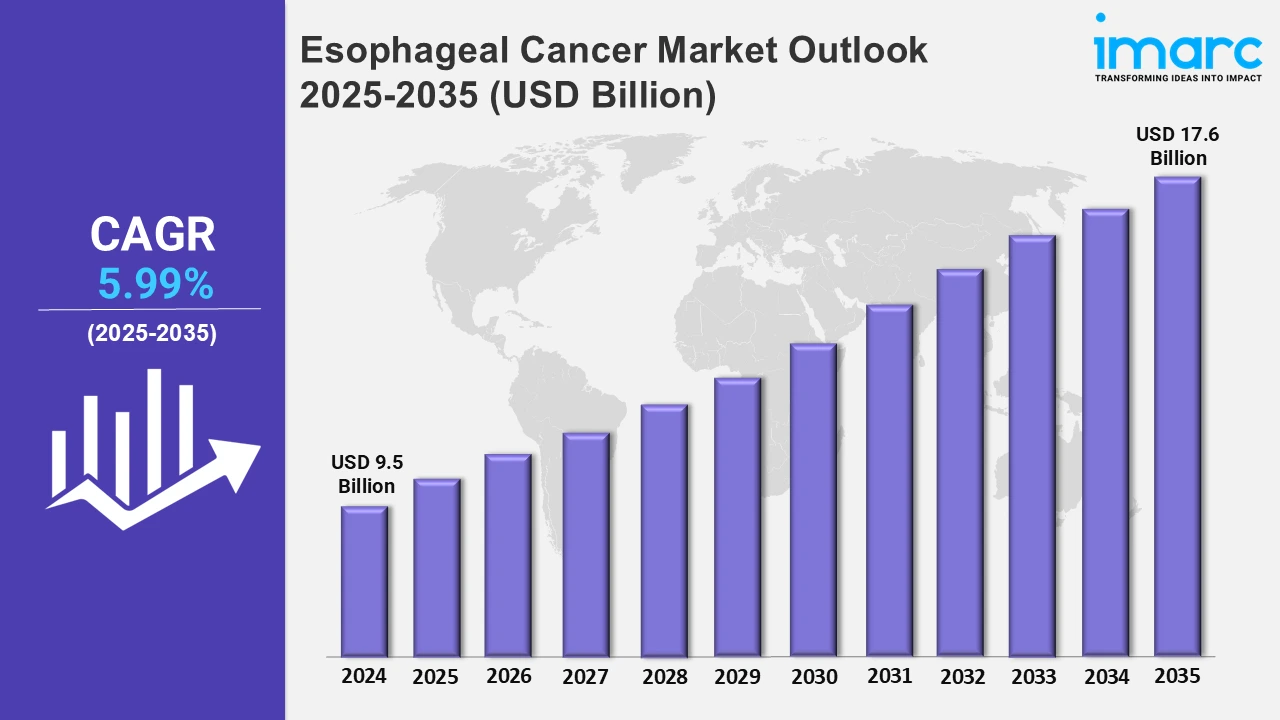
The 7 major esophageal cancer market reached a value of USD 9.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 17.6 Billion by 2035, exhibiting a growth rate (CAGR) of 5.99% during 2025-2035. The market is driven by the escalating usage of minimally invasive surgeries, such as endoscopic resection since they can reduce the risk of complications and speed up recovery time compared to traditional open procedures. Additionally, the development of targeted therapies and innovative treatments is further propelling the market growth.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Esophageal Cancer Market
The esophageal cancer market is witnessing a robust growth, owing to improvements in early detection and diagnostic technology. One of the most significant improvements is the advancement of high-resolution endoscopy and endoscopic ultrasound (EUS). Both these methods give high-resolution images of the lining of the esophagus, enabling the identification of precancerous lesions and tumors at an early stage. Narrow-band imaging (NBI) and confocal laser endomicroscopy (CLE) also augment endoscopic specificity by enhancing contrast and identifying aberrant cellular changes in real-time. Liquid biopsy and circulating tumor DNA (ctDNA) testing have become non-invasive means of early detection. Through the study of blood tests, these methods can detect tumor-specific biomarkers and genetic mutations, allowing diagnosis at an early stage before symptom onset. Liquid biopsy also applies to tracking therapy response and residual disease detection in minimal amounts, minimizing invasive treatment. Artificial intelligence (AI) and machine learning are also changing esophageal cancer diagnosis. Image analysis through AI is able to identify early-stage cancerous alterations with greater accuracy than conventional methods and helps in quicker and more accurate diagnosis. AI-based algorithms used in endoscopic machines help in real-time detection of cancer, decreasing the likelihood of misdiagnosis and enhancing patient outcomes. The application of molecular and genetic profiling also enables individualized treatment options by detecting genetic mutations and cancer biomarkers that are particular to esophageal cancer. This makes targeted treatment and immunotherapies more effective, boosting survival rates. As diagnostic technology continues to progress, the market for esophageal cancer looks to expand driven by earlier diagnoses, enhanced specificity, and optimized patient management and thus increased survival rates and optimized treatments.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The esophageal cancer market is expanding rapidly due to the development of novel therapies and pharmacological interventions with better survival rates, increased efficacy, and decreased toxicity. One of the major advances is the availability of immune checkpoint inhibitors (ICIs), like nivolumab and pembrolizumab, which have shown impressive survival advantages for advanced esophageal cancer patients. These PD-1 inhibitors function by stimulating the immune system to identify and kill cancer cells, providing a promising alternative for patients with unresectable or metastatic disease. The addition of immunotherapy to first line and adjuvant treatment regimens are revolutionizing the standard of care. In addition to immunotherapy, targeted therapies, such as HER2 inhibitors (trastuzumab) and VEGF inhibitors (ramucirumab), are playing a crucial role in improving patient outcomes. HER2-positive esophageal cancer patients can benefit from trastuzumab-based regimens, which enhance the effectiveness of chemotherapy. Similarly, anti-angiogenic agents like ramucirumab inhibit tumor blood vessel formation, slowing disease progression. Advancements in personalized medicine and biomarker-driven therapies are also contributing to market expansion. Genetic and molecular profiling of tumors enables the selection of precision therapies tailored to individual patient profiles, leading to better treatment responses and fewer side effects. With ongoing research and clinical trials exploring new drug combinations, CAR-T cell therapies, and next-generation biologics, the esophageal cancer market is poised for significant growth.
Marketed Therapies in Esophageal Cancer Market
Keytruda (Pembrolizumab): Merck & Co
Keytruda (Pembrolizumab) is a humanized antibody, or programmed death-1 (PD-1) inhibitor, used in cancer immunotherapy to treat esophageal cancer. It is delivered via slow intravenous injection. Pembrolizumab inhibits lymphocytes' PD-1 receptors, blocking the ligands that would deactivate them and prevent an immunological response. This permits the immune system to identify and eliminate cancer cells, but it also prevents a vital process that keeps the immune system from attacking the body itself.
Opdivo (Nivolumab): Bristol-Myers Squibb/Ono Pharmaceuticals
Opdivo (Nivolumab) is an anti-cancer drug employed in the treatment of esophageal cancer. Nivolumab's mechanism of action relies on its function as a monoclonal antibody that binds specifically to the PD-1 receptor on the surface of T cells, a type of white blood cell that is essential to the immune system's capacity to combat cancer. In normal circumstances, some cancer cells employ the PD-1 pathway to shield themselves from immune responses by inducing programmed death-ligand 1 (PD-L1), which interacts with the PD-1 receptor and prevents T-cell activation and growth. Nivolumab suppresses this interaction by binding to the PD-1 receptor, stopping tumor cells from evading immune recognition. This inhibition enhances T-cell reactivity, enhances the immune response against the tumor, and ultimately leads to the destruction of cancer cells.
Photofrin (Porfimer sodium): Pinnacle Biologics
Photofrin (Porfimer sodium) is a hematoporphyrin derivative prescribed to treat esophageal cancer. Porfimer-induced cellular damage results from the spread of radical reactions. Radical initiation may occur after the porfimer absorbs light and forms a porphyrin excited state. Spin transfer from porfimer to molecular oxygen may result in singlet oxygen. Subsequent radical reactions can produce superoxide and hydroxyl radicals. Tumor mortality can also occur due to ischemic necrosis caused by vascular blockage, which appears to be mediated in part by thromboxane A2 release.
Emerging Therapies in Esophageal Cancer Market
Tiragolumab: Genentech
Tiragolumab, developed by Genentech, is a novel immune checkpoint inhibitor with an intact Fc region. It acts by blocking the TIGIT (T-cell immunoreceptor with Ig and ITIM domains) protein on immune cells, preventing it from interacting with CD155 (poliovirus receptor), which reduces the immune response. This allows the immune system to more effectively fight cancer cells, such as those seen in esophageal cancer. Essentially, it acts as an immune amplifier, increasing the activity of T cells and natural killer cells against tumors.
Atezolizumab: Genentech
Atezolizumab, also known as Tecentriq by Genentech, is under clinical investigation for the treatment of esophageal cancer. The drug works by inhibiting the interaction between the protein PD-L1 (programmed cell death ligand 1) expressed on tumor cells and the PD-1 receptor on T cells. Essentially, it prevents cancer cells from inhibiting the immune system while allowing the body's immune cells to recognize and fight tumor cells. This mechanism is classed as immune checkpoint inhibitors.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Tiragolumab | Genentech | Antibody-dependent cell cytotoxicity; T lymphocyte stimulants; TIGIT protein inhibitors | Intravenous |
| Atezolizumab | Genentech | Cytotoxic T lymphocyte stimulants; Programmed cell death-1 ligand-1 inhibitors | Intravenous |
Detailed list of emerging therapies in Esophageal Cancer is provided in the final report…
Leading Companies in the Esophageal Cancer Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global esophageal cancer market, several leading companies are at the forefront of developing integrated platforms to enhance the management of esophageal cancer. Some of the major players include Merck & Co, Bristol-Myers Squibb, and Ono Pharmaceuticals. These companies are driving innovation in the esophageal cancer market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
Key Players in Esophageal Cancer Market:
The key players in the Esophageal Cancer market who are in different phases of developing different therapies are Merck & Co, Bristol-Myers Squibb, Pinnacle Biologics, Zymeworks, Eli Lilly and Company, BeiGene, Ono Pharmaceuticals, Genentech, and Others.
.webp)
Regional Analysis:
The major markets for esophageal cancer include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for esophageal cancer while also representing the biggest market for its treatment. This can be attributed to the rising incidence rates, largely attributed to risk factors such as gastroesophageal reflux disease, Barrett’s esophagus, obesity, smoking, and alcohol consumption, that are driving demand for effective diagnostic and therapeutic solutions.
Moreover, a major driver is the advancement in targeted therapies and immunotherapies. The introduction of immune checkpoint inhibitors like nivolumab and pembrolizumab has transformed treatment, offering better survival rates for patients with advanced esophageal cancer. Additionally, the use of HER2-targeted therapies (trastuzumab) and anti-angiogenic agents (ramucirumab) is enhancing treatment efficacy, contributing to market expansion.
Besides this, improved early detection and diagnostic technologies are also playing a crucial role. The increasing use of high-resolution endoscopy, liquid biopsy, and AI-driven diagnostic tools is enabling early identification of esophageal cancer, leading to better treatment outcomes. Screening programs and awareness campaigns are further promoting early diagnosis, reducing the number of late-stage cases.
Recent Developments in Esophageal Cancer Market:
- In September 2024, Genentech stated that the United States Food and Drug Administration (FDA) had approved Tecentriq Hybreza (atezolizumab and hyaluronidase-tqjs), the first and only PD-(L)1 inhibitor for subcutaneous (SC), under the skin injection for patients in the United States. Tecentriq Hybreza can be given subcutaneously in around seven minutes, whereas a typical IV infusion of Tecentriq (atezolizumab) takes 30-60 minutes.
- In March 2024, BeiGene, Ltd. announced that the U.S. FDA had approved TEVIMBRA (tislelizumab-jsgr) as a monotherapy for the treatment of adult patients with unresectable or metastatic esophageal squamous cell carcinoma who have received prior systemic chemotherapy that did not include a PD-(L)1 inhibitor.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the esophageal cancer market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the esophageal cancer market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current esophageal cancer marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












