Gout Market Size to Reach USD 6.3 Billion by 2035, Impelled by Advancements in Early Detection
Gout Market Outlook 2025-2035:
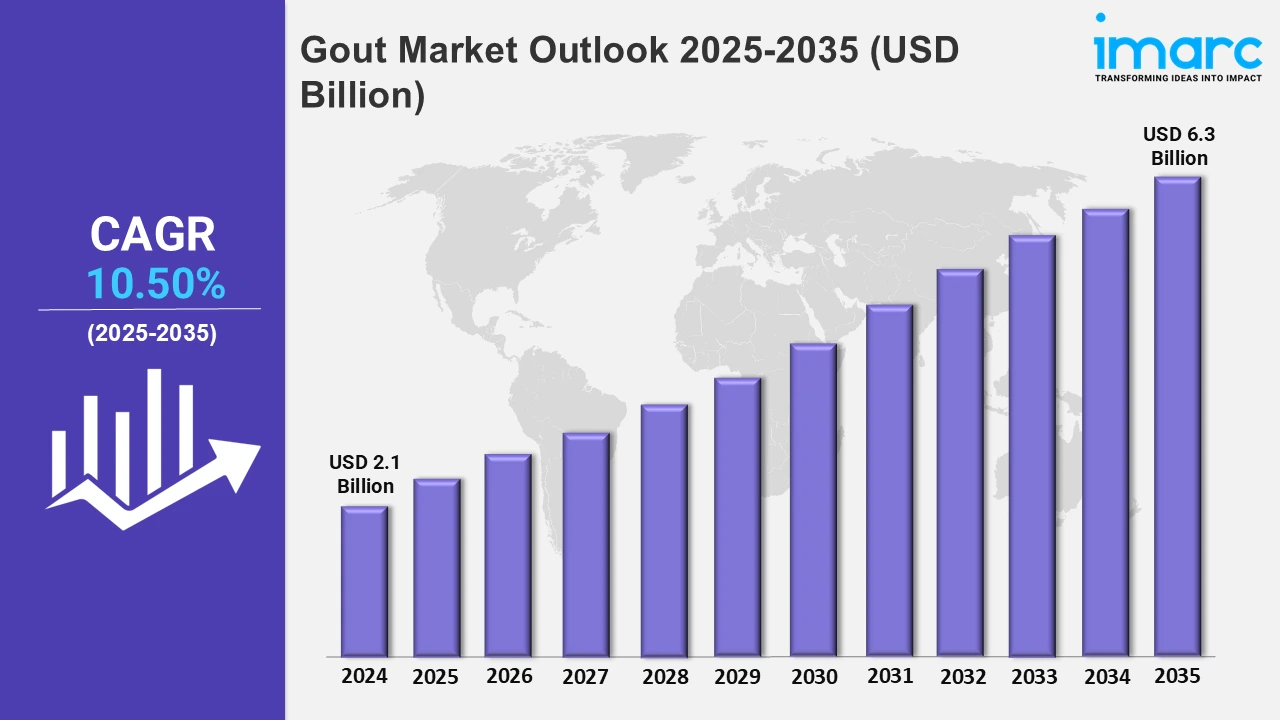
The 7 major gout markets reached a value of USD 2.1 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 6.3 Billion by 2035, exhibiting a growth rate (CAGR) of 10.50% during 2025-2035. Emerging popularity of the arthroscopic technique to treat the disease drives the market, as it easily removes uric acid deposits from the joints of patients, which in turn provides better functional outcomes. In addition, the development of targeted therapies and innovative treatments is propelling the market further.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Gout Market
The gout market is experiencing rapid expansion primarily due to advancements in early detection and diagnosis. Among the considerable developments in gout diagnosis is the advent of utilizing dual-energy computed tomography-based methods. In distinguishing gout from other diseases, DECT imaging can accurately visualize deposits of urate crystals in joints and tissues, even in asymptomatic patients. This non-invasive imaging tool is a good alternative to standard synovial fluid analysis for patients whose joints may have been aspirated unnecessarily, giving results that could be dependable. High-resolution ultrasound (HRUS) has now also become very useful in enabling the detection of monosodium urate (MSU) crystal deposits long before they present any clinical symptoms. Point-of-care (POC) devices for uric acid testing revolutionized the management of gout patients. They allow a quick uric acid test, enabling doctors to make their decisions immediately in the clinic and to start therapy right away. Furthermore, the development of some biomarkers has led to the investigation of blood and urine tests to detect early gout and monitor disease progression. AI-assisted platforms in diagnostics are enhancing the accuracy and ease of gout diagnosis. AI imaging analysis can assist in quick and precise detection of urate crystals from imaging scans and aid clinicians in fast and accurate diagnosis. Advances in technologies that enable early detection are becoming a boon for the growing gout market benefited by awareness, better patient outcomes, and adoption of advanced diagnostic solutions in clinical practice.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
With newer therapy implementations and other pharmacologic treatments, the gout market is continuing to flourish leading to the effective management of the disease, relief of symptoms, and prevention of complications. The latest innovations in pharmacotherapy are overcoming some of the restrictions imposed by older treatments like allopurinol and febuxostat, providing a more personalized and even individualized approach. The introduction of uricase-based therapies like pegloticase to lower serum uric acid levels among treatment-resistant gout patients represents a significant breakthrough in gout therapies. Pegloticase is a recombinant uricase-derived enzyme that catalyzes uric acid's conversion to allantoin, a soluble compound that facilitates easy excretion from the body. This provides rapid and long-lasting relief in very severe cases. Currently, next-generation uricase formulations are made to increase efficacy and reduce immunogenic effects in long-term treatment. Clinical studies are also under way to develop novel xanthine oxidase inhibitors that, besides allopurinol and febuxostat, will provide alternative choices to patients who do not tolerate conventional drug treatments very well. In addition, selective inhibitors of urate transporters such as URAT1 inhibitors are gaining interest in the promotion of renal uric acid excretion, hence achieving a more pronounced reduction in systemic urate burden. For instance, new biological agents aimed at the inflammatory pathways will include interleukin-1 (IL-1) inhibitors such as canakinumab, which had provided another option for the treatment of acute gout flares, thus further facilitating this market. It is expected that the gout market will continue to grow with increased patient demand for superior disease management and therapeutic outcomes as the research goes further into safer, effective, and individualized treatment strategies.
Marketed Therapies in Gout Market
Krystexxa (Pegloticase): Horizon Therapeutics plc
Krystexxa (Pegloticase) is a drug used to treat severe chronic gout that has not responded to previous treatments. The medicine works as a recombinant uricase enzyme, breaking down uric acid straight into allantoin, a water-soluble molecule that the kidneys can easily eliminate. Dissolving urate crystal accumulation in the body efficiently lowers serum uric acid levels and manages gout symptoms. This approach is performed via intravenous infusion and is distinguished from standard oral gout medicines, which largely suppress uric acid formation.
Naprelan (Naproxen sodium): Almatica
Naprelan (Naproxen sodium) is a nonsteroidal anti-inflammatory drug (NSAID) that treats acute gout and mild to moderate pain. Naproxen, like other non-selective NSAIDs, works by inhibiting COX-1 and COX-2 enzymes, which reduces prostaglandin formation. Although both enzymes contribute to prostaglandin synthesis, they have distinct functional characteristics. The COX-1 enzyme is constitutively active and can be found in normal tissues like the stomach lining, whereas the COX-2 enzyme is inducible and creates prostaglandins that cause pain, fever, and inflammation. Naproxen's intended antipyretic, analgesic, and anti-inflammatory actions are mediated by the COX-2 enzyme, whereas the COX-1 enzyme is associated with undesirable side effects such as gastrointestinal disturbance and renal toxicities.
Colcrys (Colchicine): Takeda
Colcrys (colchicine) 0.6 mg tablet is a prescription medication for adults to prevent and treat gout flare-ups. Colcrys inhibits the formation of microtubules within inflammatory cells, especially neutrophils, preventing them from migrating to the site of inflammation. This reduces the inflammatory response associated with gout episodes. The process largely interferes with the cell's capacity to move and produce inflammatory mediators such as cytokines, hence avoiding further joint damage caused by uric acid crystals in gout.
Emerging Therapies in Gout Market
AR882: Arthrosi Therapeutics
AR882, created by Arthrosi Therapeutics, is a unique uric acid-reducing substance being developed for the treatment of gout and tophaceous gout. The medicine is a potent and selective inhibitor of the uric acid transporter 1 (URAT1) protein, which is principally responsible for uric acid reabsorption in renal tubules. AR882 inhibits URAT1, which promotes uric acid excretion in urine, lowering serum uric acid levels and treating gout by reducing uric acid crystal accumulation in the body.
SEL-212: Selecta Biosciences
SEL-212 is a combination drug currently undergoing in-depth testing, designed to lower serum urate levels in patients with chronic and difficult-to-treat gout; it may, therefore, reduce irreversible tissue deposition of urate that, if unchecked, can lead to intolerable attacks of gout and malformation in the joints. With regard to its mechanism, SEL-212 consists of pegadricase, Selecta's proprietary pegylated uricase, together with ImmTOR, which acts to diminish the formation of anti-drug antibodies (ADAs). ADAs develop as a result of undesired immune responses to biologics, thus resulting in decreased therapeutic efficacy or tolerability, which is a common occurrence in several treatment modalities and in various disease states, including chronic refractory gout.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| AR882 | Arthrosi Therapeutics | SLC22A12 protein inhibitors | Oral |
| SEL-212 | Selecta Biosciences | Urate oxidase replacements | Intravenous |
Detailed list of emerging therapies in Gout is provided in the final report…
Leading Companies in the Gout Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global gout market, several leading companies are at the forefront of developing integrated platforms to enhance the management of gout. Some of the major players include Horizon Therapeutics plc, Almatica, and Takeda. These companies are driving innovation in the gout market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In December 2024, Eisai Co., Ltd. announced that it had received approval for URECE from the National Medical Products Administration in China as a treatment for gout patients with hyperuricemia.
In November 2023, Amgen revealed new data for KRYSTEXXA (pegloticase) that showed a reduction in blood pressure in adults with uncontrolled gout who are unresponsive to oral urate-lowering therapy, both with and without chronic kidney disease.
Key Players in Gout Market:
The key players in the Gout market who are in different phases of developing different therapies are Horizon Therapeutics plc, Selecta Biosciences, Almatica, Teijin Pharma, Takeda, Fuji Yakuhin, Mochida Pharmaceutical, Arthrosi Therapeutics, Dyve Biosciences, Shanton Pharma, LG Chem, and Others.

Regional Analysis:
The primary markets for gout are the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to IMARC, the USA has the largest number of gout patients and is also considered the largest market in the treatment of this disorder. This is further supported by increasing awareness among healthcare providers and patients regarding the importance of early diagnosis and continuous management of the disease to assist in better acceptance of treatment modalities.
New pharma products play a great role in shaping the industry. Whereas standard medications include allopurinol and febuxostat, immediate introduction of newer uricase-based therapeutics like pegloticase has provided a better avenue of treatment for patients who suffer from refractory gout. Apart from these treatments, there is growing interest in interleukin-1 (IL-1) inhibitors, as well as urate transporter inhibitors (URAT1 inhibitors), based on their dual action in lowering uric acid levels and controlling inflammation.
In terms of regulation, U.S. FDA support continues to favor innovation within this sector. Drug development incentives and priority review programs laid the regulatory scaffolding for the inclusion of gout treatment systems in the research portfolios of pharmaceutical companies, hence leading to more bespoke treatments.
Recent Developments in Gout Market:
- In December 2024, Arthrosi Therapeutics, Inc. revealed that it had enrolled more than 50% of patients in its pivotal Phase 3 REDUCE 2 trials, which is investigating AR882 for the lowering of serum urate (sUA) in gout.
- In July 2024, Sobi announced the commencement of a rolling Biologics License Application (BLA) to the United States Food and Drug Administration (FDA) for SEL-212. The proposal is based on the findings from the DISSOLVE I and II pivotal investigations. SEL-212 is a novel biologic medication being developed for the treatment of persistent refractory gout.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the gout market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the gout market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current gout marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












