Hodgkin’s Lymphoma Market Size to Reach USD 20.8 Billion by 2035, Impelled by Advancements in Gene Therapy and Personalized Medicine
Hodgkin’s Lymphoma Market Outlook 2025-2035:
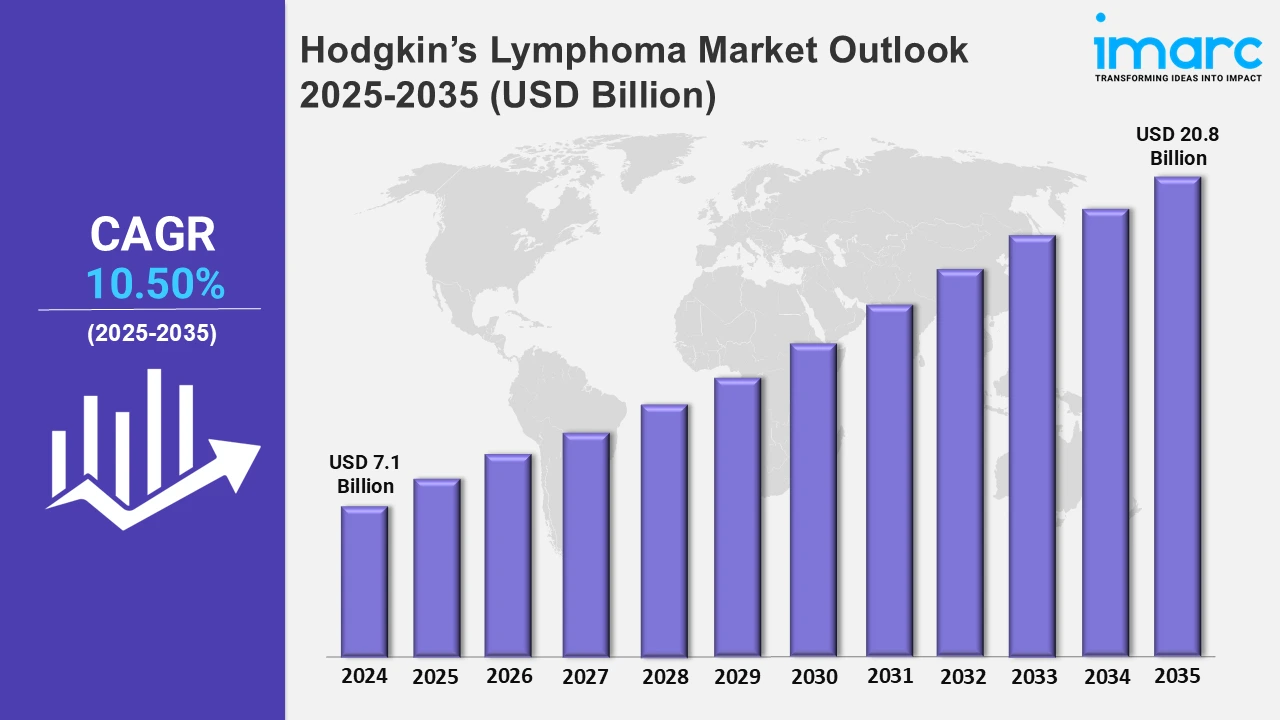
The 7 major hodgkin’s lymphoma market reached a value of USD 7.1 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 20.8 Billion by 2035, exhibiting a growth rate (CAGR) of 10.50% during 2025-2035. The market is driven by the growing use of targeted treatments, such as monoclonal antibodies, which are made especially to prevent tumor development and metastasis. Additionally, the development of innovative diagnostic techniques is further propelling the market growth.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Hodgkin’s Lymphoma Market
The Hodgkin's lymphoma market is experiencing rapid growth as a result of improvement in early detection and diagnostic technologies which are key to timely intervention and better patient outcomes. The most important development in Hodgkin's lymphoma diagnosis has been the application of positron emission tomography-computed tomography (PET-CT). PET-CT imaging allows for accurate tumor localization and metabolic analysis enabling oncologists to stage the disease correctly and measure response to therapy. PET-CT has emerged as the gold standard for Hodgkin's lymphoma surveillance enhancing early detection and avoiding unnecessary treatments. Flow cytometry and immunohistochemistry (IHC) have also transformed the diagnosis of lymphomas by allowing the thorough characterization of cancer cells. These methods allow the discrimination of Hodgkin's lymphoma from other lymphoproliferative disorders based on the identification of particular surface markers like CD30 and CD15 which are vital for targeted treatments. The advent of liquid biopsy and circulating tumor DNA (ctDNA) sequencing is also augmenting early detection further. These minimally invasive assays can detect molecular changes in blood specimens enabling real-time monitoring of disease and early relapse. New developments in artificial intelligence (AI) and machine learning are optimizing pathology processes by enhancing the accuracy of lymphoma diagnosis and prognosis prediction. Artificial intelligence-based analysis of imaging data and biopsy samples is minimizing diagnostic errors and hastening treatment decisions. With ongoing advances in diagnostic technologies Hodgkin's lymphoma detection is becoming more accurate and affordable thereby fueling market growth and enhancing survival rates through earlier and more efficient treatment interventions.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The market for Hodgkin’s lymphoma is experiencing rapid growth, driven by the emergence of innovative therapies and pharmacological treatments that enhance survival rates, minimize side effects, and provide personalized treatment approaches. A key advancement is the development of targeted therapies, notably brentuximab vedotin, an antibody-drug conjugate (ADC) that specifically targets cancer cells expressing CD30. This treatment has shown significant effectiveness in cases of relapsed and refractory Hodgkin’s lymphoma, decreasing the reliance on traditional chemotherapy. Additionally, immune checkpoint inhibitors like nivolumab and pembrolizumab have revolutionized treatment options for patients facing relapsed or refractory Hodgkin’s lymphoma. These PD-1 inhibitors function by reactivating the immune system to identify and eliminate cancer cells, resulting in sustainable remission with fewer toxic effects compared to conventional therapies. The success of immunotherapy is encouraging further research into combination approaches to enhance its effectiveness in newly diagnosed cases. Advances in CAR-T cell therapy are showing promise for Hodgkin’s lymphoma treatment. This personalized immunotherapy approach involves engineering a patient’s T cells to specifically target and eliminate cancer cells providing a potential long-term cure for patients with treatment-resistant diseases. The development of biomarker-driven precision medicine is also playing a key role in market expansion. Genetic and molecular profiling of tumors allows for the selection of therapies tailored to an individual’s disease characteristics improving outcomes and reducing unnecessary toxicity. With continuous research, FDA approvals and strong investment in novel therapeutics the Hodgkin’s lymphoma market is set for significant expansion offering patients more effective and personalized treatment options than ever before.
Marketed Therapies in Hodgkin’s Lymphoma Market
Adcetris (Brentuximab vedotin): Seagen/Takeda Oncology
When combined with vinblastine, doxorubicin, and dacarbazine, Adcetris (Brentuximab Vedotin) is authorized for use in adults with stage III or IV classical Hodgkin's lymphoma who have not received prior treatment. This medication targets the CD30 protein found on Hodgkin's lymphoma cells and delivers a cytotoxic agent known as monomethyl auristatin E (MMAE) into the cells after binding. This causes cell cycle arrest and, eventually, cell death by upsetting the microtubule network inside the cells. It functions as an antibody-drug conjugate, selectively targeting cancer cells that express CD30.
Opdivo (Nivolumab): Bristol-Myers Squibb/Ono Pharmaceuticals
Opdivo (Nivolumab) is an anti-cancer drug used for treating Hodgkin's lymphoma. T cells, which are important white blood cells in the immune system's battle against cancer, have a receptor for programmed death-1 (PD-1), which it binds to as a monoclonal antibody. By generating programmed death-ligand 1 (PD-L1), which binds to the PD-1 receptor and prevents T-cell activity and proliferation, some cancer cells take advantage of the PD-1 pathway to elude immune responses. Nivolumab blocks this interaction, allowing T cells to remain active and enhancing the immune system's ability to combat tumors, ultimately aiding in the destruction of cancer cells.
Keytruda (Pembrolizumab): Merck & Co
Keytruda (Pembrolizumab) is a humanized PD-1 inhibitor used in immunotherapy for Hodgkin lymphoma. It is administered through slow intravenous injection. Pembrolizumab blocks ligands that deactivate PD-1 receptors on cells, preventing their activation and impairing the immune response. This preserves a vital mechanism that stops the immune system from attacking healthy tissues while simultaneously allowing the immune system to identify and eliminate cancer cells.
Emerging Therapies in Hodgkin’s Lymphoma Market
AZD7789: AstraZeneca
AZD7789 is a bispecific monoclonal antibody that targets both PD-1 and TIM-3 immune checkpoints on T cells, enhancing the immune system's capability to detect and combat cancer cells in Hodgkin's lymphoma. This dual blockade aims to overcome resistance that may arise from using single checkpoint inhibitors, thus boosting T-cell activation against tumors.
AFM13: Affimed Therapeutics
AFM13 is an innovative innate cell engager designed to harness the innate immune system to target and eliminate CD30-positive hematologic cancers. By triggering natural killer (NK) cells and macrophages, it particularly promotes the killing of tumor cells that express CD30. Tetravalent bispecific innate cell engager AFM13 links innate immune cells to the tumor, allowing these immune effectors to more easily destroy cancer cells.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| AZD7789 | AstraZeneca | Antibody-dependent cell cytotoxicity; HAVCR2 protein inhibitors; Programmed cell death 1 receptor antagonists; T lymphocyte stimulants | Intravenous |
| AFM13 | Affimed Therapeutics | Antibody-dependent cell cytotoxicity; Natural killer cell stimulants | Intravenous |
Detailed list of emerging therapies in Hodgkin’s Lymphoma is provided in the final report…
Leading Companies in the Hodgkin’s Lymphoma Market:
The market analysis report from IMARC provides an in-depth review of the competitive environment within the Hodgkin’s lymphoma market. Several key companies, such as Takeda Oncology, Seagen, and Merck & Co., are leading the way in developing integrated solutions for better management of Hodgkin’s lymphoma. These organizations are fostering innovation in this sector by investing in ongoing research, enhancing diagnostic capabilities, and broadening their product lines to address the increasing demand for treatment.
In October 2023, Takeda revealed that the European Commission (EC) granted approval for ADCETRIS (brentuximab vedotin) to be used alongside doxorubicin, vinblastine, and dacarbazine (AVD) for treating adults with previously untreated CD30+ Stage III Hodgkin's lymphoma.
Key Players in Hodgkin’s Lymphoma Market:
The key players in the Hodgkin’s Lymphoma market who are in different phases of developing different therapies are Seagen, Takeda Oncology, Merck & Co, Bristol-Myers Squibb, Ono Pharmaceuticals, AstraZeneca, Affimed Therapeutics, ADC Therapeutics, Tessa Therapeutics, Genmab, and Others.

Regional Analysis:
The major markets for Hodgkin’s lymphoma include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for Hodgkin’s lymphoma while also representing the biggest market for its treatment. This can be attributed to the rising adoption of immunotherapies and targeted treatments, such as nivolumab and pembrolizumab, which offer better efficacy and fewer side effects compared to traditional chemotherapy.
Moreover, major industry players are investing heavily in R&D to introduce innovative therapies, including next-generation immunotherapies and CAR-T cell therapies. The U.S. FDA plays a crucial role in accelerating the approval of novel treatments through initiatives like orphan drug designation and breakthrough therapy status, which incentivize pharmaceutical companies to develop advanced therapies.
Besides this, the expanding healthcare infrastructure and insurance coverage in the U.S. ensures broader access to advanced diagnostics and treatment modalities. Increased awareness campaigns, patient advocacy groups, and clinical trial participation are also driving early diagnosis and better disease management.
Recent Developments in Hodgkin’s Lymphoma Market:
In December 2024, Affimed N.V. disclosed data during a poster session at the 66th ASH Annual Meeting and Exposition. This dataset comprises 22 patients from the run-in phase of the LuminICE-203 (AFM13-203) phase 2 open-label, multicenter, multi-cohort study. This trial assesses the safety and efficacy of acimtamig (AFM13) in combination with Artiva Biotherapeutics' allogeneic NK cell therapy, AlloNK, for individuals with relapsed or refractory classical Hodgkin lymphoma.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Hodgkin’s lymphoma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Hodgkin’s lymphoma market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Hodgkin’s lymphoma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












