Profitability and Cost Analysis of Sulfamethoxazole Manufacturing Plant: A Cost Model Approach
_11zon.webp)
What is Sulfamethoxazole?
Sulfamethoxazole is a common synthetic antibacterial that is a member of the sulfonamide class of antibiotics. This substance plays a very significant role in modern medicine due to the amazing capabilities offered by the substance for curing infections and bacterial diseases.
Key Applications Across Industries:
Trimethoprim and sulfamethoxazole are frequently used together to create the well-known antibiotic co-trimoxazole, which is well-known for its potency against a variety of bacterial infections. It works by preventing the manufacture of folic acid, which is necessary for the growth of bacteria. Sulfamethoxazole is an essential part of modern antibiotic therapy since it has been used to treat lung infections, urinary tract infections, and other common bacterial illnesses.
What the Expert Says: Market Overview & Growth Drivers
According to an IMARC study, the global Sulfamethoxazole market size reached USD 29.2 Million in 2024. Looking ahead, the market is expected to grow at a CAGR of approximately 3.1% from 2025 to 2033, reaching a projected value of USD 38.7 Million by 2033. The sulfonamide property of sulfamethoxazole is one of several characteristics that significantly influence this market. Being in extensive use as an antibiotic combined with trimethoprim for the treatment of bacterial infections is one such aspect.
Increasing incidents of respiratory infections, diarrheal diseases, and urinary tract infections are factors that contribute to the demand. Additionally, doctors are prescribing sulfamethoxazole-based treatments due to the increase in antimicrobial resistance (AMR), particularly for drug-resistant bacterial strains. Expansion in the market is also supported by the increasing pharmaceutical industries, especially in the developing countries. The government's efforts to enhance access to antibiotics with the addition of public health care centers facilitate the market. Furthermore, one of the main factors for sulfonamide consumption is its use in veterinary medicine for diseases in animals.
Case Study on Cost Model of Sulfamethoxazole Manufacturing Plant:
Objective
One of our clients has approached us to conduct a feasibility study for establishing a mid to large-scale sulfamethoxazole manufacturing plant in Michigan, United States.
IMARC Approach: Comprehensive Financial Feasibility
We have developed a detailed financial model for the plant's setup and operations. The proposed facility is designed with a production capacity of 3 tons of sulfamethoxazole per day.
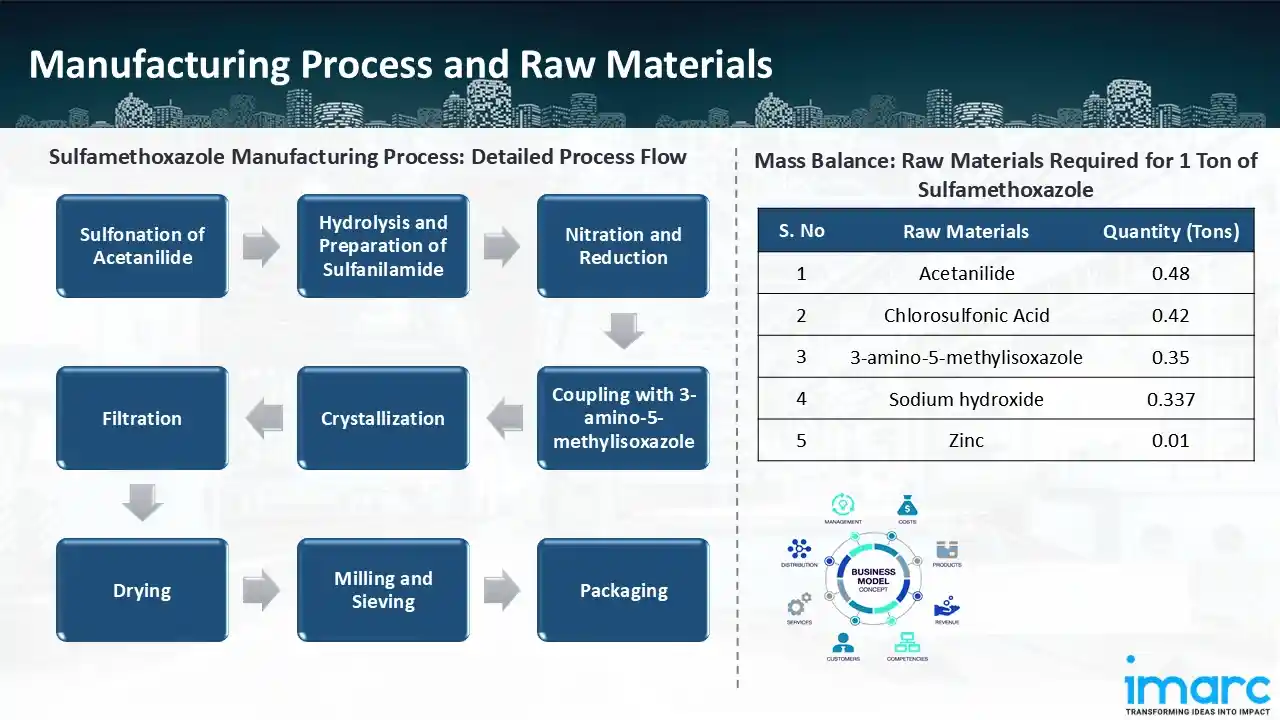
Manufacturing Process: Acetanilide undergoes a sulfonation reaction to introduce a sulfonyl functional group, which is the first step in the synthesis of sulfamethoxazole. Following hydrolysis of this intermediate, sulfanilamide—a vital component in the manufacture of sulfamethoxazole—is produced. After that, a nitro group is introduced and then reduced to create an amine group in the nitration and reduction processes. The crucial step that gives Sulfamethoxazole its unique antibacterial qualities is the subsequent coupling of this amine with 3-amino-5-methylisoxazole. The final active pharmaceutical ingredient (API) is created because of the reaction. The crude product is crystallised after synthesis to improve stability and purity. After crystallising, the chemical is filtered and dried to get rid of contaminants and leftover solvents. Following drying, the material is ground and sieved to get the appropriate particle size for formulation. After being refined, Sulfamethoxazole is packaged under carefully monitored circumstances to preserve its stability and purity before being sent to pharmaceutical companies. To guarantee adherence to legal requirements, stringent quality control procedures are implemented at every stage of the procedure.
Get a Tailored Feasibility Report for Your Project Request Sample
Mass Balance and Raw Material Required: The primary raw materials utilized in the sulfamethoxazole manufacturing plant include acetanilide, chlorosulfonic acid, sodium hydroxide, 3-amino 5-methylisoxazole, and zinc. To manufacture 1 ton of sulfamethoxazole, we require 0.48 ton of acetanilide, 0.42 tons of chlorosulfonic acid, 0.35 tons of 3-amino 5-methylisoxazole, 0.337 tons of sodium hydroxide, and 0.01 tons of zinc.
List of Machinery:
The following equipment was required for the proposed plant:
- Jacketed Reactor with Mixer and Agitator
- Storage Tank 3 KL
- 500 ltr ss 316L receiver tank
- Distillation Column 200 NB x 6 Mtr
- Liquid distributor
- Collector Cum Distributor
- Packing Support
- Solider packings
- Rotary Vacumm Dryer 2KL
- 24-inch Filter Press
- Agitated Nutsche Filter Dryer
- Receiver- 50 Ltr.
- Receiver- 100 Ltr.
- Condenser- 2 m2
- Water Ring Vacuum Pump- 5 HP
- Crystallizer 5 KL
- Manhole with cover
- inlet and outlet
- spare
- Light Glass and Slight Glass
- Coil Inlet and Outlet
- SS-316L Condenser- 7 m2
- Inlet and Outlet
- Drain
- Vent
- Utility Inlet and Outlet
Techno-Commercial Parameter:
- Capital Investment (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
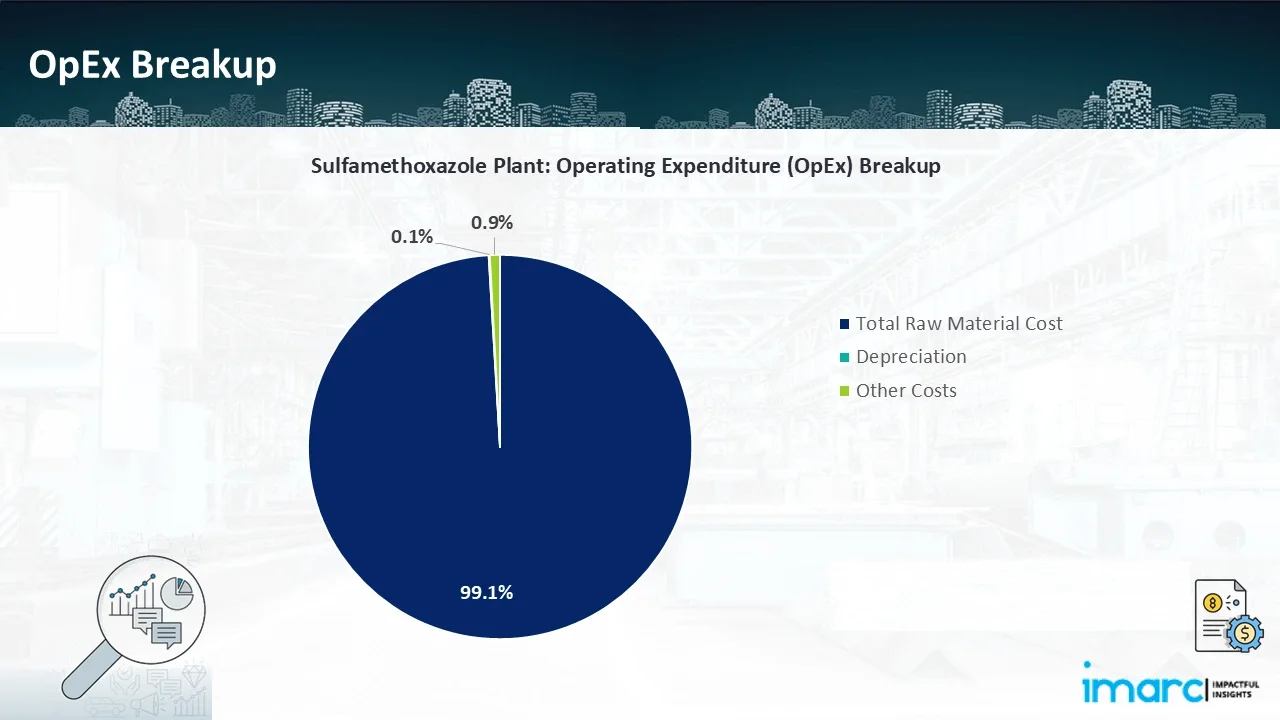
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. OpEx in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth.
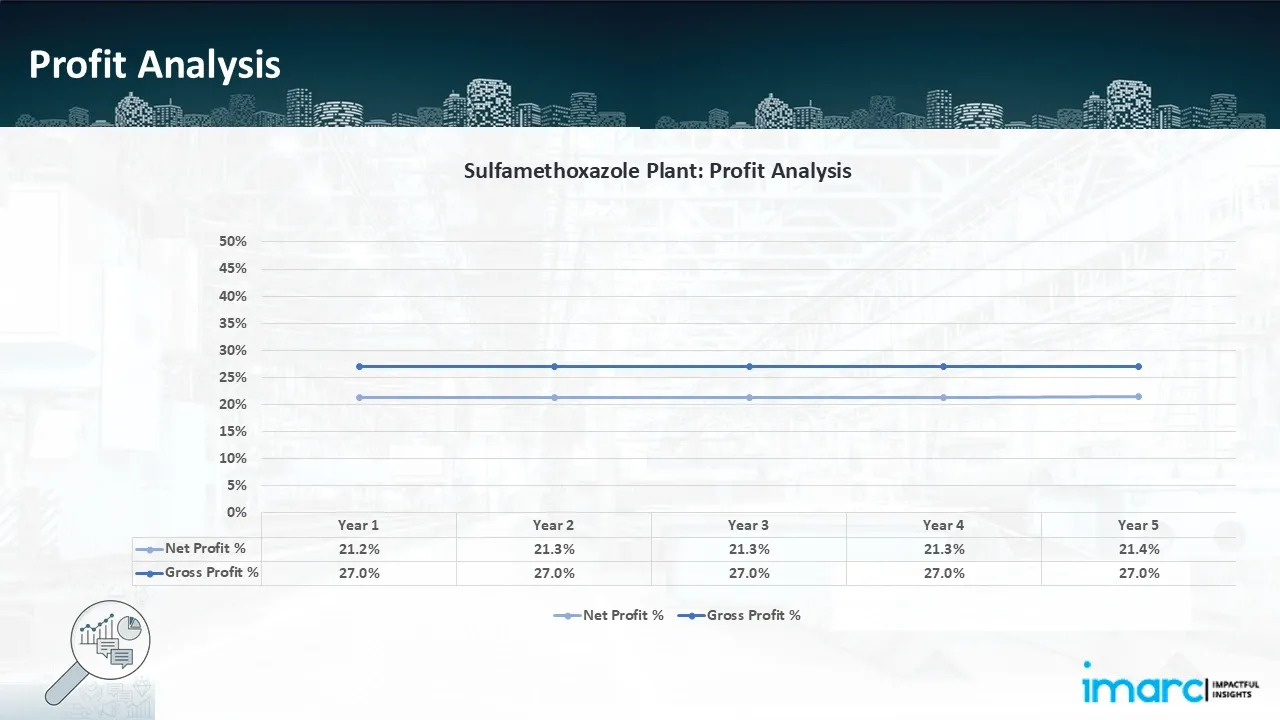
- Profitability Analysis Year on Year Basis: The proposed sulfamethoxazole plant, with a capacity of 3 tons of sulfamethoxazole per day, achieved an impressive revenue of USD 700 million in its first year. We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the projected period. Moreover, gross profit stood the same throughout the years at 27.0%, and net profit rise from 21.2% to 21.4%, highlighting strong financial viability and operational efficiency.
Conclusion & IMARC's Impact:
Our sulfamethoxazole manufacturing plant's financial model was meticulously modelled to satisfy the client's requirements. It provided a thorough analysis of production costs including capital expenditures, manufacturing processes, raw materials, and operating costs. The model predicts profitability while accounting for market trends, inflation, and any shifts in the price of raw materials. It was created especially to satisfy the demand of producing 3 tons of sulfamethoxazole per day. Our commitment to offering precise, client-cantered solutions that ensure the long-term success of significant industrial projects by giving the client useful data for strategic decision-making is demonstrated by this comprehensive financial model.
Why Choose IMARC:
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Brief List of Our Services: Market Entry and Expansion
- Market Entry and Opportunity Assessment
- Competitive Intelligence and Benchmarking
- Procurement Research
- Pricing and Cost Research
- Sourcing
- Distribution Partner Identification
- Contract Manufacturer Identification
- Regulatory Approvals, and Licensing
- Factory Setup
- Factory Auditing
- Company Incorporation
- Incubation Services
- Recruitment Services
- Marketing and Sales
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104











