Menorrhagia Market Size to Reach USD 1,435.0 Million by 2035, Impelled by Growing Adoption of Hormonal and Non-Hormonal Therapies
Menorrhagia Market Outlook 2025-2035:
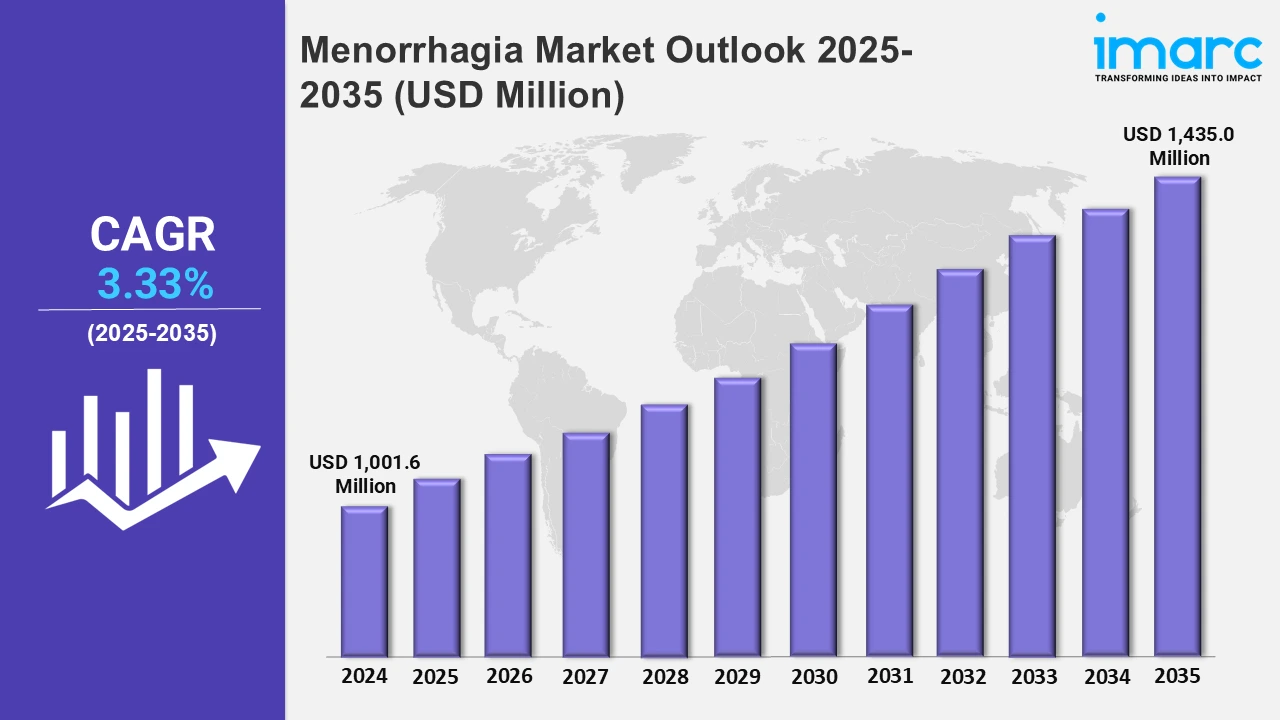
The menorrhagia market reached a value of USD 1,001.6 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 1,435.0 Million by 2035, exhibiting a growth rate (CAGR) of 3.33% during 2025-2035. The treatment landscape for menorrhagia is experiencing a significant shift, moving beyond mere symptom management to embrace targeted therapies that address the underlying causes of heavy menstrual bleeding. Historically, remedies dealt more with ways to mitigate the effects of excessive bleeding and used forms of hormonal treatments and dilation and curettage (D&C). But with the appreciation of better-known contributing factors, such as fibroids in the uterus, endometrial irregularities, and hormonal imbalances, there has been a shift toward more advanced treatments that directly attack these problems. This marks the transition from the treatment of the symptom to the treatment of the disease. This paradigm shift is reflected in the increasing popularity of minimally invasive procedures. The preference for endometrial ablation and uterine artery embolization over traditional invasive surgeries has been rising, with effective solutions targeted specifically to the site of disease along with the added advantage of quicker recovery periods. Apart from this, advances in pharmaceuticals also are very important. Tranexamic acid, hormonal intrauterine devices (IUDs), and numerous oral contraceptives are now expanding the repertoire for the treatment of menorrhagia, providing women with a wider range of choices tailored to their specific needs and circumstances.
To get more information on this market, Request Sample
Rising Awareness and Early Diagnosis in Menorrhagia
There has been an increased interest in patient education programs and sensitization campaigns, greatly contributing to early recognition and management of menorrhagia. With women increasingly being knowledgeable about symptoms and potential complications of heavy menstrual bleeding, more have flocked to the hospital at much earlier stages, allowing for quicker and positive diagnoses. The causes of menorrhagia such as uterine fibroids, polyps, or endometrial abnormalities are identified through advanced diagnostic tools such as ultrasound, hysteroscopy, and MRI. This is crucial because the treatment would be based on the specific diagnosis of the causes of menorrhagia. Advanced diagnostic tools enable healthcare providers to conduct non-invasive, precise evaluations, which are crucial for tailoring treatment plans to the specific needs of the patient. On the other hand, the increased vigilance of health care providers toward screening for menorrhagia, has driven early diagnosis and treatment. This rise in awareness and diagnostic accuracy is fostering a shift toward personalized treatments, ensuring that women receive the most effective interventions for their unique conditions. Ultimately, these advancements in early diagnosis are improving patient outcomes and empowering women to take control of their reproductive health.
Strategic Collaborations and Investments Transforming the Menorrhagia Treatment Landscape
The landscape of menorrhagia treatment is undergoing a profound transformation, fueled by a surge in strategic collaborations and investments within the pharmaceutical industry. Pharmaceutical companies are increasingly forging partnerships to accelerate the development of novel treatments, recognizing the significant unmet need for effective and targeted therapies. Such collaborations often bridge the gap between well-established pharmaceuticals and agile biotech firms by synergizing each other's research, development, and commercialization strengths to approach the complex causative factors of heavy menstrual bleeding. The development of such collaborative ecosystems is further helping the industry streamline regulatory pathways while expanding market reach, which ultimately enables fast innovation of more effective, targeted treatments for women suffering from menorrhagia. This proactive approach is transcending symptomatic treatment, where the development of therapies that deal with underlying conditions like uterine fibroids and endometrial abnormalities are in focus. Additionally, another notable trend is seen wherein the big pharmaceutical companies are investing heavily in biotech companies focused entirely on women's health. The capital is very critical to further the progress of novel therapeutic strategies, such as genetic therapies and individualized treatment protocols. This strategic financial investment fuels the research and development of cutting-edge treatments while also marking a significant shift in the pharmaceutical industry's focus on women's health, further signaling a promising future for those seeking advanced and tailored solutions for menorrhagia. This combined momentum from strategic collaborations and financial investments is significantly accelerating progress in the field, offering hope for more effective and personalized treatments for those suffering from this debilitating condition.
Marketed Therapies in the Menorrhagia Market
Lysteda (Tranexamic acid-controlled release) - Amring Pharmaceuticals
Lysteda (tranexamic acid-controlled release) by Amring Pharmaceuticals is a key therapeutic option for managing menorrhagia. It works by reducing excessive menstrual bleeding through the inhibition of fibrinolysis, stabilizing blood clots. This targeted treatment offers an effective, non-hormonal solution for women suffering from heavy menstrual bleeding.
Leading Companies in the Menorrhagia Market:
The treatment landscape of menorrhagia is experiencing a dynamic shift, with the pharmaceutical industry's commitment to innovations and high investment. The leading pharmaceutical giants, such as Amring Pharmaceuticals and others, are actively developing advanced therapies and more personalized approaches in the management of this challenging condition. Their efforts include all possible options- from well-known hormonal and non-hormonal medications to very innovative options, such as tranexamic acid and hormonal IUDs. This diversification is a result of a much deeper understanding of the various causes of menorrhagia that can allow each patient to have a more precisely targeted approach in her care. One of the most prominent trends in this evolution is the increasing focus on precision medicine and the study of combination therapies, which reflects the complexity of menorrhagia. These new approaches are designed not only to reduce symptoms but also to enhance long-term reproductive health, improve quality of life, and provide more sustainable solutions for women who experience heavy menstrual bleeding.
Key Players in the Menorrhagia Market:
The key players in the Menorrhagia market who are in different phases of developing different therapies are Amring Pharmaceuticals and others.

Regional Analysis:
The treatment landscape for menorrhagia is significantly changing, largely driven by the pharmaceutical industry's strong commitment to innovation and significant financial investment. Ferring Pharmaceuticals, Bayer, and AbbVie are leading this transformation with significant resources dedicated to developing advanced therapies tailored to address the complexities of heavy menstrual bleeding. From traditional hormonal treatments and non-hormonal medications to more innovative solutions like tranexamic acid and hormonal intrauterine devices (IUDs), their work revolves around a number of treatment options, ones that better manage the condition of menorrhagia. At its heart lies the industry's focus on precision medicine, an approach that hopes to tailor treatments to each patient's distinct needs and conditions. Along with this, there is an increasing interest in combination therapies, which reflect a collaborative approach to address the diverse factors contributing to menorrhagia, such as uterine fibroids or endometrial abnormalities. These strategic developments ensure the alleviation of these symptoms and the betterment of reproductive health without complications such as anemia, thus bringing an overall improvement in the quality of life for women who suffer from menorrhagia. This comprehensive, individualized approach represents a major step forward in menorrhagia management, offering new hope and improved outcomes for those affected.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the menorrhagia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the menorrhagia market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current menorrhagia-marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












