Opioid Use Disorder Market Size to Reach USD 4.6 Billion by 2035, Impelled by The Development of Novel Extended-Release Medications
Opioid Use Disorder Market Outlook 2025-2035:
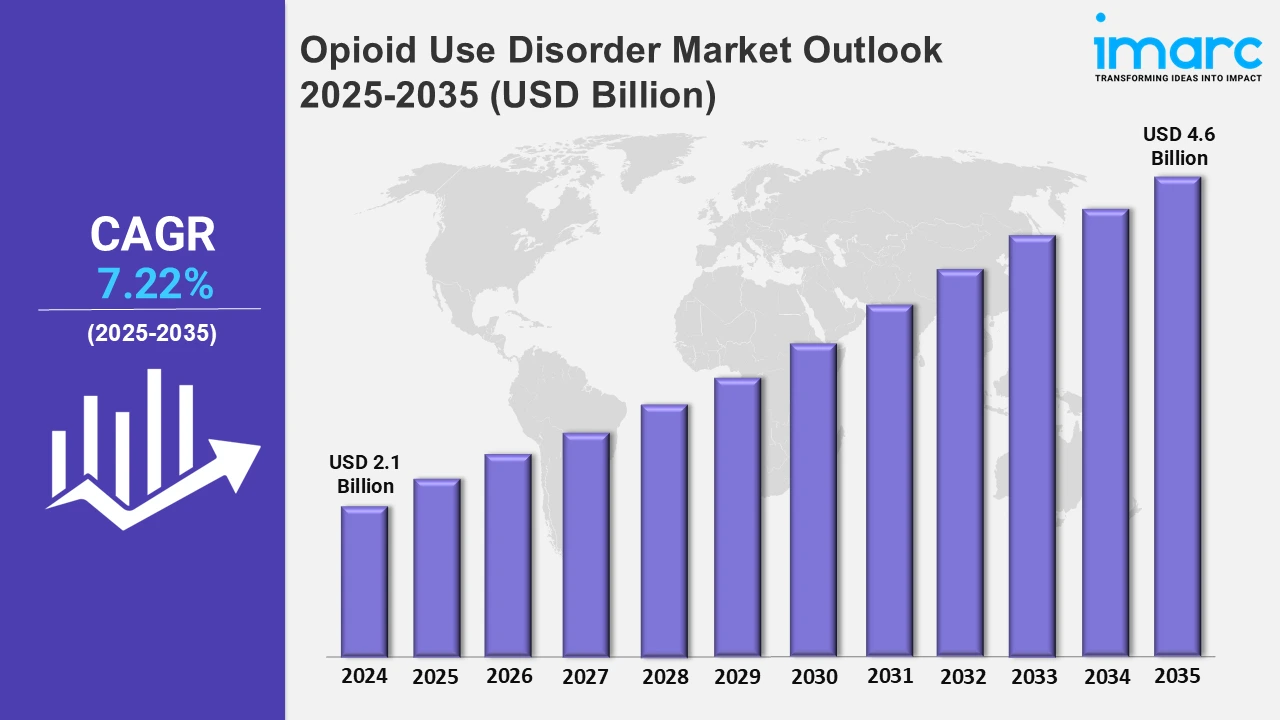
The 7 major opioid use disorder market reached a value of USD 2.1 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 4.6 Billion by 2035, exhibiting a growth rate (CAGR) of 7.22% during 2025-2035. The Opioid Use Disorder (OUD) market is driven by the rise in demand for non-invasive and minimally invasive treatment options as more individuals become interested in transcranial magnetic stimulation, digital therapeutics, and wearable medical devices that offer an effective solution in helping withdrawal symptoms and craving, thereby increasing patient engagement, and adherence. Advanced techniques focused on neurological pathways for addiction reduce dependency on long-term pharmacological treatments while improving outcomes of treatment. They provide better comfort, fewer side effects, and less stigma than traditional treatments, hence increasing the appeal for patients seeking novel and personalized recovery alternatives.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Opioid Use Disorder Market
Modern diagnostic and treatment technologies are remarkably transforming the Opioid Use Disorder (OUD) market to improve patient management and outcomes. Functional MRI (fMRI) and PET scans directly visualize and monitor brain activity, which is relevant to addiction and withdrawal symptoms. This helps doctors assess and create a treatment plan more accurately. These advancements are supported by biomarker-based diagnostics, such as genetic and epigenetic profiling, that identify individual susceptibility to opioid addiction to facilitate intervention through personalization. Molecular diagnostics, such as NGS and pharmacogenetic testing, are becoming increasingly important in the detection of genetic variations that influence opioid metabolism and responsiveness to treatment, thereby guiding more effective MAT. The inclusion of artificial intelligence in the diagnostic framework has improved precision in the prediction of relapse threats, optimization of drug dosages, and tailoring of behavioral therapies, with fewer requirements for subjective assessments. Non-invasive treatments, such as TMS, VNS, and CBT in virtual reality settings, have reduced side effects while involving patients more in their treatment. Novel wearable technologies, including smart biosensors and patches, enable real-time monitoring of physiological and behavioral parameters, providing instant recognition of withdrawal symptoms or relapse triggers and facilitating timely interventions. These technologies are particularly beneficial in remote or underserved areas, ensuring continuous care and improved long-term outcomes.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The market for OUD is expanding due to newly introduced therapies in combination with advanced pharmacological treatments. Other new pharmacological agents are coming up that target opioid withdrawal symptoms, cravings, and nervous system imbalances that may happen with long term dependence on opioids. Such programs have been tested and proved efficient with less side effects and having a good mechanism, thereby increasing the satisfaction of the patient and creating better clinical outcomes. Biologic drugs are also being rapidly researched for the treatment of moderate to severe cases of OUD, especially among those with chronic neurological and inflammatory conditions associated with the prolonged misuse of opioids. Examples include monoclonal antibodies aimed at opioid-related pathways, like dynorphin and inflammatory cytokines, in regulating the reward system of the brain and reduction of neuroinflammation upon withdrawal. This development in drug delivery systems is attainable through sustained-release injectables, implantable buprenorphine formulations, and nanotechnology-based carriers, which enable localized and long-lasting drug delivery and thus maintain therapeutic drug levels with reduced dosing frequency and risk of misuse. Adjunct therapies are also under development to restore the natural balance of the gut-brain axis and the overall recovery. Included are probiotics and immunomodulators, which are involved in stabilizing the gut microbiome in relation to mood regulation and substance use behavior. Combination therapies involving opioid antagonists, such as naltrexone, and anti-inflammatory or cognitive enhancers, promise to address the multiple pathophysiological mechanisms of OUD, including craving suppression and cognitive restoration. Other increasingly used non-invasive pharmaceutical formulations include transdermal patches and biofilm-disrupting agents in implantable formulations, as they are easy to apply, reduce systemic exposure, and are patient-centric. This is changing the OUD management landscape and providing long-term outcome in recovery.
Marketed Therapies in Opioid Use Disorder Market
Lucemyra (Lofexidine): Britannia Pharmaceuticals
Lucemyra (lofexidine hydrochloride) is an FDA-approved, non-opioid medication used to manage withdrawal symptoms in patients discontinuing opioid use. It works by reducing the release of norepinephrine, thereby alleviating symptoms like anxiety, sweating, and agitation associated with opioid withdrawal.
Vivitrol (Naltrexone controlled-release): Alkermes
Vivitrol (naltrexone extended-release injectable) is a once-monthly medication used to treat opioid use disorder by blocking opioid receptors in the brain, thereby preventing the euphoric and sedative effects of opioids. It is particularly effective for maintaining abstinence after detoxification, with the added benefit of being non-addictive and reducing the risk of misuse compared to daily medications.
Subutex (Buprenorphine extended-release): Indivior
Subutex (buprenorphine) is a partial opioid agonist used in the treatment of opioid use disorder (OUD). It helps reduce cravings and withdrawal symptoms without producing the full euphoric effects of opioids, making it an effective option for medication-assisted therapy (MAT).
Emerging Therapies in Opioid Use Disorder Market
INDV-2000: Indivior
INDV-2000 is an investigational non-opioid treatment for Opioid Use Disorder (OUD) developed by Indivior PLC. It functions as a selective orexin-1 receptor antagonist, targeting neural pathways associated with addiction. In June 2024, the first subject was dosed in a Phase 2 double-blind, placebo-controlled study to assess its safety and efficacy over three months in individuals with moderate to severe OUD.
AZD4041: AstraZeneca
AZD4041 is an orexin-1 receptor antagonist that was under investigation by AstraZeneca as an adjunctive treatment for moderate to severe opioid use disorder (OUD). However, the development of AZD4041 was discontinued after it demonstrated a drug-drug interaction with the antifungal itraconazole, leading to the termination of its Phase 2 clinical trial.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| INDV-2000 | Indivior | Orexin receptor type 1 antagonists | Oral |
| AZD4041 | AstraZeneca | Orexin receptor type 1 antagonists | Oral |
Detailed list of emerging therapies in Opioid Use Disorder is provided in the final report.
Leading Companies in the Opioid Use Disorder Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Opioid Use Disorder market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Opioid Use Disorder. Some of the major players include Britannia Pharmaceuticals, Indivior, Alkermes, AstraZeneca, and others. These companies are driving innovation in the Opioid Use Disorder market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Opioid Use Disorder.
In June 2024, Indivior has administered the first dose in a Phase II clinical trial of INDV-2000 for individuals with opioid use disorder (OUD). This double-blind, placebo-controlled, randomized, dose-ranging study is designed to assess the safety and efficacy of the treatment over three months in participants with moderate to severe OUD who are seeking treatment. The trial will include individuals who have recently started or completed short-term medically supervised opioid withdrawal using transmucosal (TM) buprenorphine and are considering a non-opioid treatment option.
Key Players in Opioid Use Disorder Market:
The key players in the Opioid Use Disorder market who are in different phases of developing different therapies are Britannia Pharmaceuticals, Indivior, Alkermes, AstraZeneca, Barr Pharmaceuticals, Kinoxis Therapeutics, ATAI Life Sciences/DemeRx, MediciNova, and Others.
Regional Analysis:
Major markets for Opioid Use Disorder include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to IMARC projections, the United States possesses the biggest patient pool for Opioid Use Disorder while being the biggest market for its treatment. Recently, novel treatments for Opioid Use Disorder have been developed in the field. Advancements in opioid antagonists, neuromodulation therapies, and digital therapeutics aim at solving the core challenges of OUD: craving, withdrawal, and imbalances in the neurological system. Breakthrough formulations, extended-release injectables, and implantable devices bypass the constraints of old-fashioned treatments by ensuring sustained drug delivery, thereby improving patient adherence toward a healthier lifestyle and resulting in better outcomes with minimal side effects.
With advances in diagnostic tools and methodologies, OUD severity and contributing factors, such as genetic predisposition and neurochemical imbalances, can be identified earlier and more accurately. Such innovations allow for timely and targeted treatment approaches that reduce the risk of relapse and minimize adverse events. Other drivers in the OUD market are regulatory approvals, increased investment in research and development, and growing collaborations between pharmaceutical companies, diagnostic technology providers, and research institutes. AI-based diagnostic tools and telemedicine platforms are also facilitating care delivery in remote and underserved geographies, thus democratizing access to cutting-edge treatments. Advanced therapies and diagnostic solutions continue to drive innovation in North America and Europe, propelling the Opioid Use Disorder market toward sustained growth and better patient outcomes.
Recent Developments in Opioid Use Disorder Market:
- In May 2023, the U.S. Food and Drug Administration approved Brixadi (buprenorphine) extended-release injection for subcutaneous administration as a treatment for moderate to severe opioid use disorder (OUD). Brixadi is available in two formulations, a weekly injection for patients who have initiated treatment with a single dose of a transmucosal buprenorphine product or those already receiving buprenorphine treatment, and a monthly injection for patients who are currently on buprenorphine therapy.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Opioid Use Disorder market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Opioid Use Disorder market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Opioid Use Disorder marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.


 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












