Global Pediatric Vaccines Market Anticipated to Reach USD 72.7 Billion by 2033 - IMARC Group
Global Pediatric Vaccines Market Statistics, Outlook and Regional Analysis 2025-2033
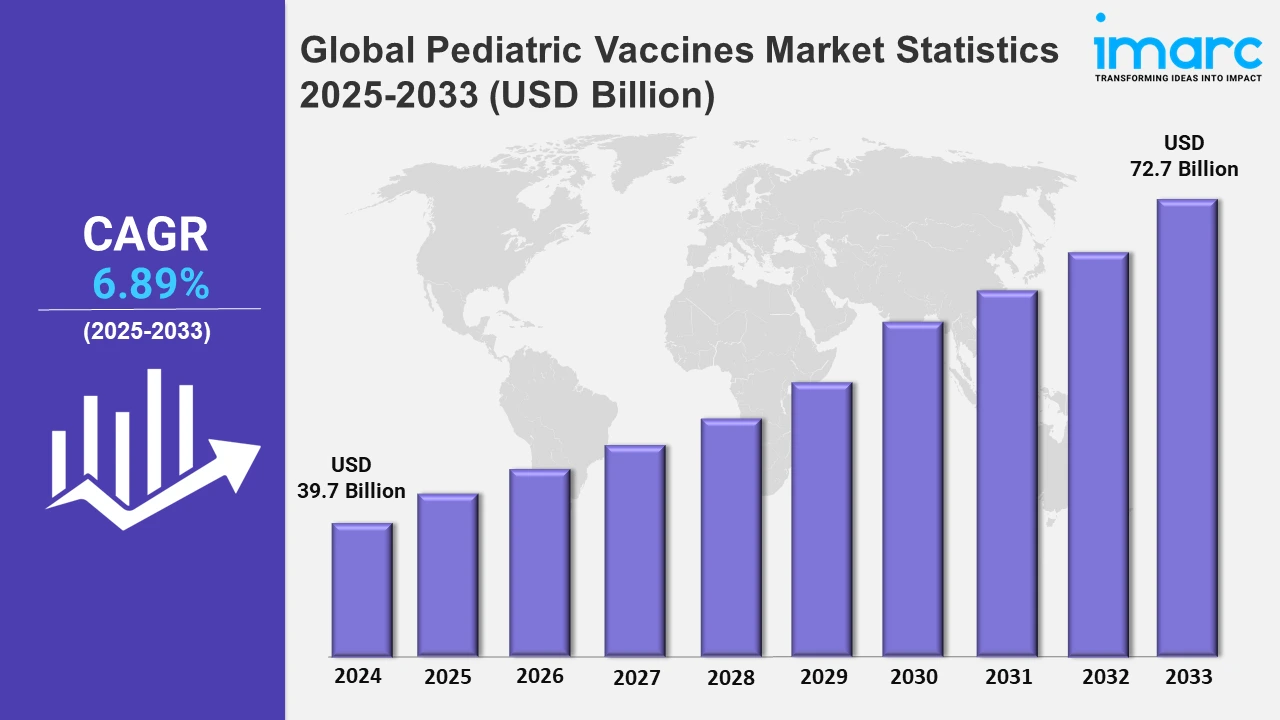
The global pediatric vaccines market size was valued at USD 39.7 Billion in 2024, and it is expected to reach USD 72.7 Billion by 2033, exhibiting a growth rate (CAGR) of 6.89% from 2025 to 2033.
To get more information on the this market, Request Sample
Health organizations across the globe are increasingly focusing on vaccination drives to reduce child mortality rates. Public health initiatives and campaigns by groups, such as the World Health Organization (WHO) and UNICEF, aiming to inform communities about the importance of pediatric vaccines are gaining momentum, especially in developing economies. Approximately 560,000 children less than ten years of age were immunized with polio vaccine during the first round of a 3-phased, 12-day emergency vaccination campaign conducted in the Gaza Strip by WHO in three phases from 1st to 12th September 2024. The campaign vaccinated 558,963 children with nOPV2 after thorough planning and coordination. This entailed the widespread application of teams vaccinating at selected fixed sites at health facilities and outreach posts. These campaigns are favoring the growth of the overall market, supported by collaborations between governments and non-governmental organizations (NGOs) to increase access to vaccines. Additionally, the increasing awareness regarding vaccines for children due to the widespread use of social media and other digital platforms that distribute crucial information quickly, are helping parents make informed decisions for their children. Educational programs in schools and clinics reinforce this awareness, promoting timely vaccinations and the benefits of immunization schedules. These collective efforts are enhancing immunization rates globally.
Increasing funding and the implementation of favorable policies from governments across the globe are propelling the market. Subsidies and financial aid programs make vaccines more accessible, especially in developing countries, where affordability is a significant concern. Policies promoting universal vaccination coverage and mandatory immunization for school admissions are creating a structured approach to vaccination, ensuring higher participation. For instance, The Centers for Disease Control and Prevention (CDC) published the 2024 immunization schedules on 16th November 2023, approved by the American Academy of Pediatrics. Key updates include new entries and safety precautions for RSV-mAb (nirsevimab) for infants up to 8 months and high-risk children up to 19 months, detailing immunization timing and considerations for different RSV seasonality, RSVPreF (Abrysvo) for pregnant individuals, and mpox vaccine (Jynneos) for adults at risk. Headers were revised to “Vaccines and Other Immunizing Agents” to include monoclonal antibodies. Governments of various countries are also aligning their efforts with international health bodies and pharmaceutical companies to enhance the network of delivering vaccines. Moreover, the partnership is strengthening cold chain infrastructure and logistics, ensuring that even the most remote locations are able to access vaccines. With the growing amount of support in line with policy-driven mandates, the shift in the global demand and distribution dynamics of pediatric vaccines is also rising.
Global Pediatric Vaccines Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share on account of robust healthcare infrastructure, widespread awareness, and significant government funding.
North America Pediatric Vaccines Market Trends:
Widespread campaigns by different departments of the government and health care providers are sensitizing individuals across North America about the importance of childhood vaccinations against numerous infectious diseases. Favorable insurance policies and reimbursement structures also relieve some burden on the parents and the caretakers, resulting in higher rates of inoculation. The region has been actively taking initiatives and is motivating more individuals to access the vaccines by offering programs such as VFC for children in the U.S. Similarly, Pfizer Inc. and Valneva SE announced that they have finalized enrollment of the Phase 3 trial of its Lyme disease vaccine candidate, VLA15, called Vaccine Against Lyme for Outdoor Recreationists, or VALOR on 4th December 2023. VALOR is based on the positive results of the previous Phase 1 and 2 trials and aims to include adults and children of both genders to cover efficacy, safety, lot consistency, and immunogenicity of VLA15. This combination of infrastructure, funding, innovation, and policy support ensures that North America is an imperative driving force in this market for pediatric vaccines.
Asia-Pacific Pediatric Vaccines Market Trends:
The Asia-Pacific region is witnessing significant growth due to higher government efforts, expanding healthcare structures, and increasing awareness among parents. The immunization program in the region is gaining momentum, especially with respect to the diseases preventable by vaccines. International aid and collaborations are being undertaken for such programs in countries including India and China. With children comprising the largest demographic in the region, and in light of ongoing efforts to improve the accessibility and affordability of vaccines, manufacturers are strategically prioritizing this sector as a critical area of focus.
Europe Pediatric Vaccines Market Trends:
Europe remains a strong market for pediatric vaccines, bolstered by well-established healthcare systems and mandatory immunization policies in many countries. High awareness levels among the population and comprehensive government-backed vaccination programs ensure consistent demand. Additionally, substantial funding for research and development (R&D) activities supports the introduction of new and improved vaccines. Western Europe, led by countries such as Germany, France, and the UK, plays a significant role, while Eastern Europe is gradually increasing its vaccine coverage and access.
Latin America Pediatric Vaccines Market Trends:
The market in Latin America is growing as governments enhance healthcare outreach and immunization programs. Countries such as Brazil and Mexico are implementing strategic campaigns to improve vaccination rates, supported by partnerships with global health organizations. Challenges such as logistical issues and economic disparities persist but improving healthcare infrastructure and focused public health initiatives are propelling access. Regional collaborations and awareness campaigns are gradually enhancing immunization coverage and driving market growth across Latin America.
Middle East and Africa Pediatric Vaccines Market Trends:
The market in the Middle East and Africa faces challenges due to economic disparities and limited healthcare infrastructure in certain areas. However, support from international organizations and partnerships with NGOs are promoting immunization efforts. Governments in more developed nations within the region, such as Saudi Arabia and the UAE, are investing in extensive healthcare initiatives to increase the overall vaccination rates. Expanding outreach programs and foreign aid are critical factors helping to enhance immunization coverage in less developed regions.
Top Companies Leading in the Pediatric Vaccines Industry
Some of the leading pediatric vaccines market companies include Bio-Med (P) Limited, Daiichi Sankyo Company Limited, GSK plc, Indian Immunologicals Limited, Merck & Co. Inc., Panacea Biotec, Pfizer Inc., and Sanofi, among others. The US International Development Finance Corporation decided to grant Panacea Biotec a long-term loan of up to $20 million on 12th September 2024, so that the firm may expand the capacity of its hexavalent vaccine. With the help of DFC's funding, Panacea will be able to quickly finish the current expansion and provide the hexavalent vaccine to UN organizations for kid vaccination across the world.
Global Pediatric Vaccines Market Segmentation Coverage
- On the basis of the type, the market has been categorized into multivalent and monovalent, wherein multivalent vaccines represent the leading segment. Their dominance is attributed to their ability to protect against multiple diseases within a single dose. This feature enhances convenience and compliance, particularly important in regions with limited access to healthcare. They simplify immunization schedules, reducing the number of injections required and associated logistical challenges. Their efficiency in preventing multiple infections at once drives their widespread adoption, making them a preferred choice for national immunization programs and contributing to the market's strong growth.
- Based on the technology, the market is classified into conjugate, live attenuated, inactivated, subunit, toxoid, and others, amongst which conjugate dominates the market. This can be attributed to their proven effectiveness in eliciting a strong immune response, especially in young children. By linking antigens to carrier proteins, these vaccines enhance the body's ability to recognize and combat bacteria and viruses. Their success in preventing diseases like Haemophilus influenzae type B (Hib) and pneumococcal infections is driving the widespread use. Governments and health organizations favor conjugate vaccines in immunization programs, solidifying their position as the leading technology in the market.
- On the basis of the application, the market has been divided into infectious diseases, cancer, allergy, and others. The infectious disease segment is supported by widespread immunization programs aimed at preventing diseases, such as polio, measles, and influenza. The cancer segment is growing as vaccines such as HPV gain prominence for cancer prevention. Allergy vaccines, while still developing, are beginning to capture attention due to increasing pediatric allergy rates.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 39.7 Billion |
| Market Forecast in 2033 | USD 72.7 Billion |
| Market Growth Rate (2025-2033) | 6.89% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Types Covered | Multivalent, Monovalent |
| Technologies Covered | Conjugate, Live Attenuated, Inactivated, Subunit, Toxoid, Others |
| Applications covered | Infectious Disease, Cancer, Allergy, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Bio-Med (P) Limited, Daiichi Sankyo Company Limited, GSK plc, Indian Immunologicals Limited, Merck & Co. Inc., Panacea Biotec, Pfizer Inc., Sanofi, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












