Peripheral T-cell Lymphoma Market Size to Reach USD 1,051.2 Million by 2035, Impelled by Advancements in Diagnostics
Peripheral T-cell Lymphoma Market Outlook 2025-2035:
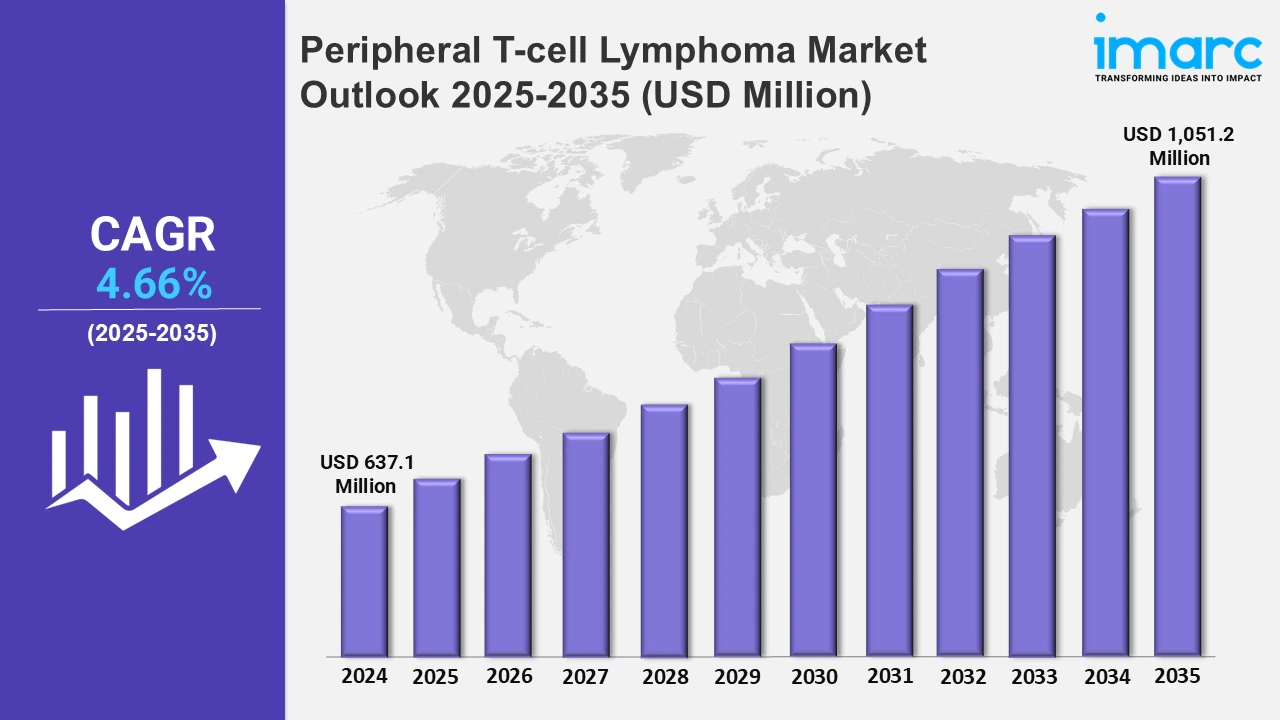
The 7 major Peripheral T-cell Lymphoma markets reached a value of USD 637.1 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 1,051.2 Million by 2035, exhibiting a growth rate (CAGR) of 4.66% during 2025-2035. The market expansion for Peripheral T-cell Lymphoma (PTCL) is growing because of enhanced disease recognition innovative treatment methods and current research on disease biological elements. The rising diagnostic initiatives along with innovative therapeutic approaches such as immune checkpoint inhibitors and new chemotherapy options, are driving the market demand. Personalized medicine and biological treatments have started to become popular because they create additional treatment possibilities. Additionally, drug development speed receives momentum through collaborations together with governmental support which is contributing to the market expansion. Multiple conditions suggest that the PTCL market is experiencing sustained growth according to current trends.
To get more information on this market, Request Sample
Advancements in Diagnostics: Driving the Peripheral T-cell Lymphoma Market
The development of the peripheral T-cell lymphoma (PTCL) market is significantly expanding, driven by recent advancements in diagnostic technology. The accuracy and speed of PTCL diagnosis have increased through NGS and liquid biopsy innovations which enable early detection and improved disease identification. Medical research through advanced methods reveals extensive information about PTCL genetics and molecules therefore enabling better disease classification along with prognosis prediction. Moreover, immunohistochemistry (IHC) combined with flow cytometry has increased the identification of distinct tumor markers which leads to a better understanding of PTCL subtype classification. The improved diagnostic processes enable doctors to establish precise early diagnoses with complete confidence. The PTCL subtypes identification process also benefits from artificial intelligence (AI) and machine learning (ML) which analyze complex data sets to deliver fast and accurate results. These evolving technologies enable better insights into the disease which leads to improved disease monitoring and assessment thus driving the growth of the PTCL market.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
New therapies and pharmacological treatments are the prime motivators in driving the peripheral t-cell lymphoma treatment expansion into the markets. Research centers mainly work upon innovating novel advances in treatments with targeted therapy and immunotherapies, both to control and manage these multiple forms of PTCLs. The market looks for enhanced treatments, such as immune checkpoint inhibitors monoclonal antibo,dies and small molecule inhibitors, because these therapies hold promise for improving outcomes in challenging-to-treat cases. Furthermore, increasing research activity and funding interest in clinical trials of innovative therapies is propelling the market demand. The enhanced comprehension of PTCL molecular processes enables the development of individualized effective treatments that expand treatment possibilities and drives the market expansion.
Marketed Therapies in Peripheral T-cell Lymphoma Market
Beleodaq (Belinostat): Acrotech Biopharma
Beleodaq (Belinostat) represents a histone deacetylase inhibitor which Acrotech Biopharma developed as an approved medicine against relapsed or refractory peripheral T-cell lymphoma (PTCL). Beleodaq prevents enzyme action that controls gene expression to slow down the growth of cancer cells. Patients with PTCL receive intravenous Beleodaq as a medication that demonstrates effectiveness in treating patients who did not respond to previous treatments.
Adcetris (Brentuximab vedotin): Seagen/Takeda Oncology
Adcetris (Brentuximab vedotin) is an antibody-drug conjugate approved for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL). The drug attacks cancer cell CD30 receptors while delivering chemotherapy agents to stop tumor growth. The treatment drug Adcetris shows excellent results in PTCL patients notably among patients who no longer benefit from standard medical approaches. The medical staff administers this drug through intravenous delivery.
Istodax (Romidepsin): Celgene Corporation
Istodax (Romidepsin) is an FDA-approved drug created by Celgene Corporation to treat peripheral T-cell lymphoma (PTCL) patients who experience relapse or non-response to previous treatments. The drug changes how genes express themselves thus stopping cancer cells from multiplying. The medical data shows that Istodax (Romidepsin) effectively treats peripheral T-cell lymphoma patients whose disease has not responded to previous treatment approaches. Patients receive Istodax through a process of intravenous infusion.
Emerging Therapies in Peripheral T-cell Lymphoma Market
Copiktra (Duvelisib): Secura Bio
Copiktra (Duvelisib) exists as an investigational oral drug that blocks both PI3K-delta and PI3K-gamma to disrupt pathways by which cancer cells multiply. Medical experts employ this drug for treating peripheral T-cell lymphoma (PTCL). The drug allows control over immune cell signaling to achieve tumor growth reduction and better clinical benefits in patients dealing with this serious condition.
AZD 4205 (Golidocitinib): Dizal Pharmaceutical
AZD 4205 (Golidocitinib) represents an experimental drug that inhibits both JAK1 and JAK3 enzymes for peripheral T-cell lymphoma (PTCL) treatment. Golidocitinib functions by blocking both JAK1 and JAK3 enzymes that control the signaling process which activates immune cells and triggers inflammatory responses. Golidocitinib inhibits enzymatic activity to slow down malignant T-cell multiplication and control immune-driven inflammation to improve treatment results for patients with peripheral T-cell lymphoma. The pharmaceutical industry tests the drug within clinical research to determine its safety profile and treatment performance.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Copiktra (Duvelisib) | Secura Bio | Phosphatidylinositol 3 kinase delta inhibitors; Phosphatidylinositol 3 kinase gamma inhibitors | Oral |
| AZD 4205 (Golidocitinib) | Dizal Pharmaceutical | Dizal Pharmaceutical | Oral |
Detailed list of emerging therapies in Peripheral T-cell Lymphoma is provided in the final report…
Leading Companies in the Peripheral T-cell Lymphoma Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global peripheral T-cell lymphoma market, several leading companies are at the forefront of developing integrated platforms to enhance the management of peripheral T-cell lymphoma. Some of the major players include Takeda Oncology, Daiichi Sankyo, Celgene Corporation, and others. These companies are driving innovation in the Peripheral T-cell Lymphoma market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for peripheral T-cell lymphoma.
Key Players in Peripheral T-cell Lymphoma Market:
The key players in the peripheral T-cell lymphoma market who are in different phases of developing different therapies are Onxeo, Seagen, Takeda Oncology, Acrotech Biopharma, Astex Pharmaceuticals, Secura Bio, Affimed Therapeutics, Celgene Corporation, Daiichi Sankyo, Dizal Pharmaceutical, and others.

Regional Analysis:
The major markets for peripheral T-cell lymphoma include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest market size for the treatment of peripheral T-cell lymphoma since it has the largest pool of patients suffering from peripheral T-cell lymphoma. Recent advancements in peripheral T-cell lymphoma, show that the research in the field has largely shifted towards improving diagnosis techniques, including gene expression profiling and next-generation sequencing in a better definition of subtypes for appropriate therapy. Some new immunotherapies, such as immune checkpoint inhibitors, have shown efficacy in clinical trials in refractory PTCL. Other targeted therapies for specific mutations and pathways are gaining ground. In addition, CAR-T cell therapy has demonstrated promising results in PTCL treatment and holds a great deal of promise for relapsed or refractory patients. Ongoing research is also looking into the role of epigenetic modifications in the development of PTCL and treatment response.
Recent Developments in the Peripheral T-cell Lymphoma Market:
- In December 2024, Secura Bio presented a poster at the 2024 American Society of Hematology (ASH) Annual Meeting in San Diego, CA. The poster showcased new findings from the company’s Phase 2 PRIMO trial, highlighting the use of duvelisib in the treatment of relapsed or refractory (R/R) peripheral T-cell lymphoma (PTCL).
- In June 2024, Daiichi Sankyo announced that EZHARMIA (valemetostat tosilate) was approved in Japan for the treatment of adult patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). EZHARMIA becomes the first dual inhibitor targeting both EZH1 and EZH2 enzymes to be approved for PTCL, following its receipt of the SAKIGAKE designation for this indication.
- In January 2023, Secura Bio announced that it had been awarded Orphan Drug Designation in Europe for Duvelisib to treat patients diagnosed with Peripheral T-cell Lymphoma. This designation of the drug has the potential to effectively address a rare, serious condition and will provide the company with incentives such as market exclusivity and financial benefits for further development.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the peripheral T-cell lymphoma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the peripheral T-cell lymphoma market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current peripheral T-cell lymphoma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












