Psychosis Market Size to Reach USD 17.6 Billion by 2035, Impelled by Rising Prevalence of Psychotic Disorders
Psychosis Market Outlook 2025-2035:
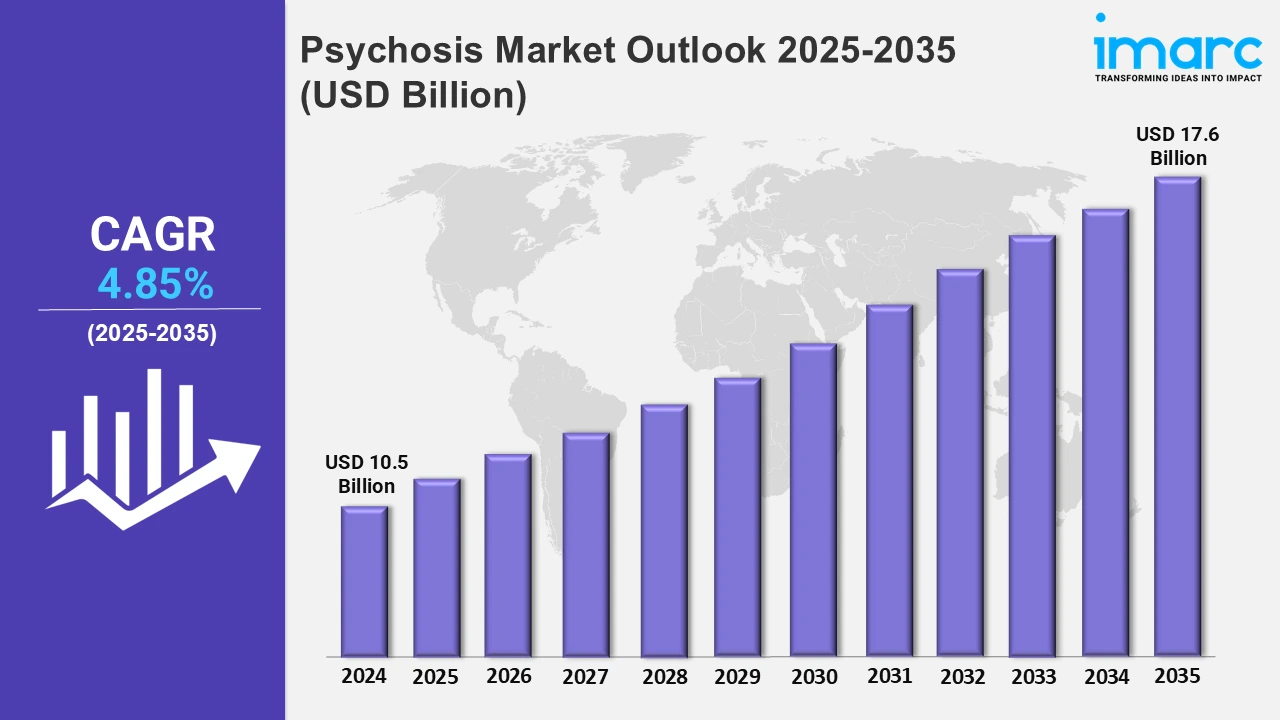
The 7 major psychosis market reached a value of USD 10.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 17.6 Billion by 2035, exhibiting a growth rate (CAGR) of 4.85% during 2025-2035. The psychosis market is growing highly, mainly because of the growing diagnosed cases and awareness of the condition. There has been progress in diagnostic equipment and increased awareness that has resulted in earlier diagnosis and treatment. Improved efficacy with lesser side effects in new antipsychotic medications is the most important driver of this growth. Government support and mental health initiatives are also contributing, along with increasing investments in research and development for better therapeutic solutions. The market is further shaped by the growing demand for personalized treatments and long-acting injectable therapies. In addition, the rise of telemedicine and digital mental health platforms is making treatment more accessible and convenient for patients. This comprehensive approach is helping to address the needs of a wider patient base, pushing the psychosis market to expand rapidly.
To get more information on this market, Request Sample
Rising Prevalence of Psychotic Disorders: Driving the Psychosis Market
The psychosis market is growing in line with the increases observed in psychotic disorders, such as schizophrenia, bipolar disorder, and severe depression. As this trend becomes more common, the need for effective treatments rises- medications, therapeutic interventions, and support systems-in aging populations and in urban areas with more diagnoses. New drugs especially the novel antipsychotics are developed to reduce the side effects, thus growing the market. Another factor contributing to the rising demand in the market is enhanced awareness and early diagnosis, which allows them to manage their conditions well. The rise in psychotic disorders among younger populations also calls for specialized treatments. Moreover, global health organizations and governments are prioritizing mental health, which has led to greater investments in research and development, further supporting the growth of the psychosis market.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The psychosis treatment market is primarily growing due to the discovery of new therapies and pharmacological treatments. As scientific studies about the biological causes of psychotic disorders are being advanced, researches are currently focusing on creating more effective treatment interventions that target specific disorders such as schizophrenia and bipolar disease. The introduction of newer drug classes, such as atypical antipsychotics and agents that target both dopamine and serotonin receptors, is improving treatment outcomes while reducing side effects seen with older medications. In addition, the emergence of personalized medicine with the support of pharmacogenomics is allowing doctors to give better customized treatments with better results. Early intervention has also gained greater importance, particularly in the initial stages of psychosis, which delays the progression of the condition. More awareness regarding mental health and reduced stigma associated with it also increases the demand for these new treatments. These factors and new drug mechanism studies are some factors that might bring about significant growth in this market for treating psychosis, along with an intent on improving patients' quality of life and prevention of relapse.
Marketed Therapies in Psychosis Market
Abilify (Aripiprazole): Bristol-Myers Squibb/Otsuka Pharmaceutical
Abilify (Aripiprazole) is an atypical antipsychotic medication used to treat conditions like schizophrenia, bipolar disorder, and major depressive disorder. It works by modulating dopamine and serotonin receptors in the brain to help reduce symptoms of psychosis, such as hallucinations, delusions, and disorganized thinking. Abilify helps restore balance to neurotransmitters, improving mood, cognition, and behavior in patients with psychotic disorders.
Risperdal (Risperidone): Janssen
Risperdal (Risperidone), developed by Janssen, is an atypical antipsychotic medication used to treat conditions like schizophrenia and bipolar mania. It functions by regulating neurotransmitters, such as dopamine and serotonin, in the brain. This helps to reduce psychotic symptoms, including delusions, hallucinations, and agitation, while also enhancing mood and cognitive function.
Zyprexa (Olanzapine): Eli Lilly and Company
Zyprexa (Olanzapine) developed by Eli Lilly and Company is an atypical antipsychotic used to treat schizophrenia and bipolar disorder. It helps restore the balance of neurotransmitters in the brain, reducing symptoms such as hallucinations, delusions, and mood swings. Zyprexa is available in oral and injectable forms for acute and long-term management of psychosis.
Emerging Therapies in Psychosis Market
NBI-1117568: Neurocrine Biosciences
NBI-1117568 (NBI-568) is an investigational, oral, muscarinic M4 selective agonist developed by Neurocrine Biosciences for the treatment of schizophrenia and psychosis. It targets the muscarinic M4 receptor to improve cognitive and behavioral symptoms while potentially reducing side effects associated with traditional dopamine-based therapies. The drug has shown promising results in Phase 2 clinical trials for adults with schizophrenia and related psychotic disorders.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| NBI-1117568 | Neurocrine Biosciences | Muscarinic M4 receptor agonists | Oral |
Detailed list of emerging therapies in Psychosis is provided in the final report…
Leading Companies in the Psychosis Market:
The market research report by IMARC includes a profound analysis of the competitive landscape present in the market. Various dominant companies operating around the globe have been on a spree to advance the integrated psychosis management platform present in the market. A few of the companies include Bristol-Myers Squibb, Eli Lilly and Company, Otsuka Pharmaceutical, among others. These companies are driving psychosis market innovation through constant research, diagnostic tools, and growing products for psychosis market that meets the growing demand.
Key Players in Psychosis Market:
The key players in the psychosis market who are in different phases of developing different therapies are Bristol-Myers Squibb, Janssen, Sunovion Pharmaceuticals, Vanda Pharmaceuticals, ACADIA Pharmaceuticals, Eli Lilly, Karuna Therapeutics, Luye Pharma, Otsuka Pharmaceutical, Neurocrine Biosciences, Sumitomo Pharma, and others.
_11zon.webp)
Regional Analysis:
Major markets for psychosis are in the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. In line with IMARC's estimates, the patient population for psychosis is highest in the United States and, concurrently, is its biggest market in terms of therapy. New breakthroughs in the area of psychosis have concentrated their efforts on strategies of early diagnosis and treatment. Advances in brain mapping have identified essential areas, the hippocampus first among them, where psychosis-related changes may take their start; earlier interventions at this stage open up new treatment possibilities. Therapies involving avatars were developed to bring patients face to face with a controlled virtual situation in which distressing voices confront them, effectively reducing symptoms of psychosis. Early intervention programs targeting individuals who are experiencing their first episode of psychosis are also proving effective in improving long-term outcomes, thus underlining the importance of timely and specialized care. These breakthroughs are changing the landscape of psychosis treatment, offering hope for more effective management and better patient outcomes.
Recent Developments in Psychosis Market:
- In September 2024, the U.S. Food and Drug Administration approves Cobenfy, which has the combination of xanomeline and trospium chloride, as an oral capsule preparation for adults that treats schizophrenia, which is an antipsychotic drug unlike all other predecessors since its long use standard-of-care, since it works targeting the cholinergic receptors while other antipsychotic medications are focused at dopamine receptors.
- In August 2024, Neurocrine Biosciences conducted a Phase 2 clinical study on NBI-1117568, or NBI-'568, in adults suffering from schizophrenia. The drug demonstrated positive results in the clinical trial. NBI-'568 is the first experimental oral muscarinic M4 selective agonist being developed to treat schizophrenia.
- In June 2024, Luye Pharma announced that it had received an Establishment Inspection Report from the U.S. Food and Drug Administration (FDA), confirming that the manufacturing facility for Paliperidone Palmitate Extended-Release Injectable Suspension (LY03010) successfully passed a Pre-Approval Inspection (PAI). The inspection resulted in a No Action Indicated (NAI) status, meaning no FDA-483 observations were issued.
- In April 2024, Vanda Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval for Fanapt (iloperidone) to treat psychosis in adults, particularly for acute manic and mixed episodes associated with bipolar I disorder. This approval was based on findings from a Phase 3 clinical trial, which was randomized, double-blind, and placebo controlled.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the psychosis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the psychosis market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current psychosis marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












