Surgical Site Infections Market Size to Reach USD 1,238.5 Million by 2035, Impelled by Escalation in Surgical Procedures
Surgical Site Infections Market Outlook 2025-2035:
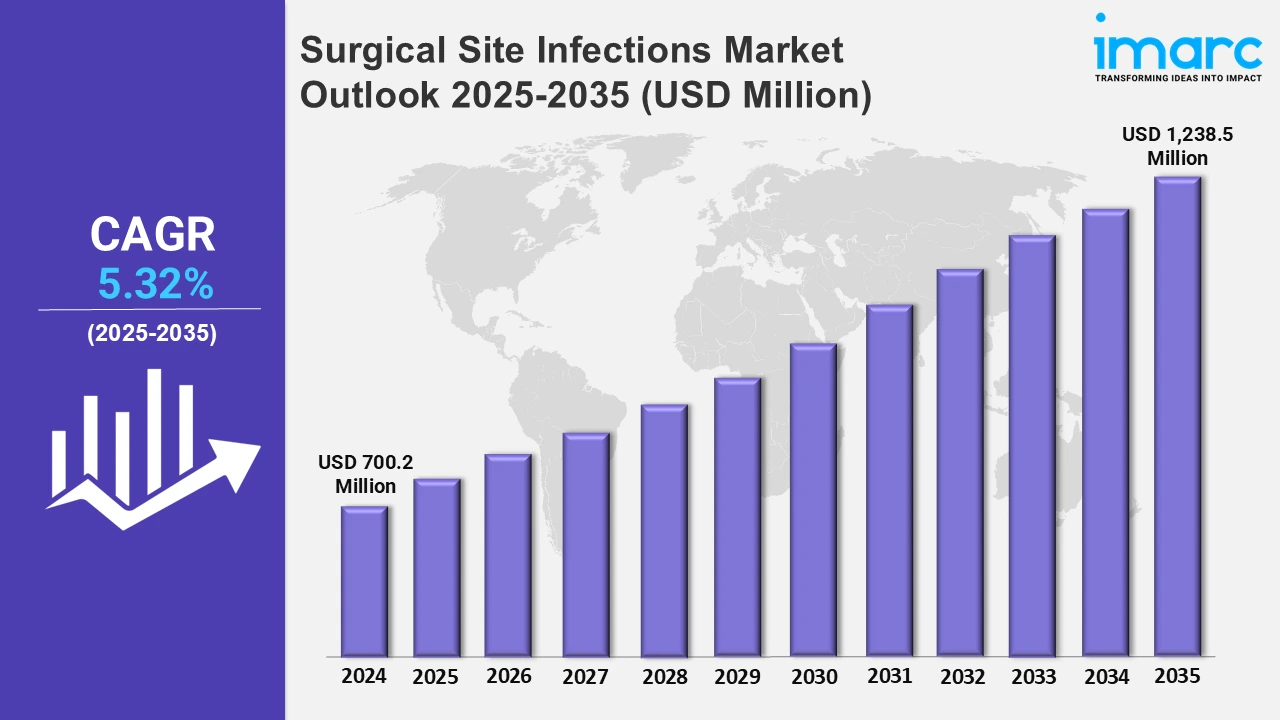
The 7 major surgical site infections market reached a value of USD 700.2 Million in 2024. Looking forward, the IMARC group expects the 7MM to reach USD 1,238.5 Million by 2035, exhibiting a growth rate (CAGR) of 5.32% during 2025-2035. The market for Surgical Site Infections is growing rapidly with the increasing number of surgeries being performed and higher awareness about infection prevention. The demand for antimicrobial treatments, wound care products, and sterilization solutions is driving growth in this category. Furthermore, the focus on patient safety, along with best infection control practices in healthcare premises, is another factor that boosts market growth. Increased healthcare expenditure and better development of healthcare infrastructures, primarily in developing economies, are factors that are increasing the market share.
To get more information on this market, Request Sample
Escalation in Surgical Procedures: Driving the Surgical Site Infections
The most prominent reason for the increase in surgical site infections (SSIs) is the rise in surgical procedures. With more surgeries being performed, especially complex and high-risk ones, the chances of infections grow due to factors like longer surgery times, invasive techniques, and patients with pre-existing health conditions. Individuals with conditions such as diabetes, obesity, or weakened immune systems are particularly vulnerable to infections after surgery. In addition, implant and prosthetic use in certain procedures creates a new pathway for bacteria, making SSIs more likely. Greater hospital stays and intensive post-operative care increase exposure to infections obtained in healthcare. Elective procedures, such as cosmetic and weight-loss surgeries, increase the number of patients exposed. Poor sterilization practices, antibiotic resistance, and contamination of the hospital environment add to the complexity of this issue. To overcome these challenges, there should be a greater emphasis on enhancing infection prevention practices, better sterilization techniques, and more effective antimicrobial treatments to reduce the occurrence of SSIs.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
New therapies and pharmacological treatments are contributing greatly to the growth of the surgical site infections (SSIs) market. With a fast-paced increase in SSIs, particularly after surgery, the need for appropriate treatment is also on the rise. There is a constant development of new antibiotics, wound care solutions, and other specialized treatments that would meet the continuously rising demand. The development of research in microbes has been a highlight in the development of better-fitting treatments to combat antibiotic resistance, which is a major factor in managing SSIs. Beyond the traditional antibiotics, there exist innovations such as biologics, which contain antimicrobial peptides and immune-modulating drugs that expand the portfolio of treatment alternatives for infections which are hard to treat. Nanotechnology has been incorporated into products used in wound care, decreasing infection rates but allowing for fast recovery based on stimulation of tissue regeneration at sites of surgery. As the healthcare sector is shifts more toward prevention of infection and better recovery of patients post-surgery, these advanced therapies are benefitting the patients' outcome while also reducing hospital stays and associated healthcare expenses. As awareness about SSIs grows and research continues to make strides, these novel therapies are expected to further boost the market for SSI treatments, driving its expansion in the coming years.
Emerging Therapies in Surgical Site Infections Market
D PLEX: Polypid
-PLEX by PolyPid is a more advanced treatment for preventing surgical site infections (SSIs). It uses the company's PLEX technology to deliver a sustained, localized release of doxycycline at the surgical site, which maintains high antibiotic levels for up to 30 days. The controlled delivery system helps to reduce infection risks, including those from antibiotic-resistant bacteria, especially for abdominal colorectal surgeries.
XF 73: Destiny Pharma
XF-73 is an innovative therapeutic agent for Phase 2 trials, targeting surgical site and staphylococcal infections. The substance alters bacterial cell membrane permeability, thus better absorbing antimicrobial agents. Disruption of bacterial membranes by XF-73 increased bacteria susceptibility to antibiotics, thus fighting antibiotic resistance and decreasing the post-surgical infection rates.
E 101: Exoxemis
E-101 Solution developed by Exoxemis also called Zempia, is a topical antiseptic that is used to prevent surgical site infections. It works through myeloperoxidase (MPO) to deliver broad microbicidal activity against gram-negative and gram-positive bacteria, viruses, spores, yeast, and fungi. In fact, E-101 Solution is effective against antibiotic-resistant bacteria and inactivates endotoxins, which may help prevent sepsis.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| D PLEX | Polypid | Protein 30S ribosomal subunit inhibitors | Topical |
| XF 73 | Destiny Pharma | Cell membrane permeability modulators | Topical |
| E 101 | Exoxemis | Reactive oxygen species stimulants | Topical |
Detailed list of emerging therapies in Surgical Site Infections is provided in the final report…
Leading Companies in the Surgical Site Infections Market:
The IMARC market research report provides an in-depth analysis of the competitive structure of the market. Across the global surgical site infections market, there are various top companies working to create integrated platforms for better management of surgical site infections. Some of the major players include Polypid, and others. These companies drive innovation in the surgical site infections market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for surgical site infections.
Key Players in Surgical Site Infections Market:
The key players in the surgical site infections market who are in different phases of developing different therapies are Destiny Pharma, Exoxemis, Polypid, and others.

Regional Analysis:
The major markets for surgical site infections include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for surgical site infections while also representing the biggest market for its treatment. Recent advancements in the treatment of surgical site infections (SSIs) have led to the development of cutting-edge techniques to improve recovery and reduce infection risks. One key innovation is the creation of biodegradable sutures that emit electrical signals when the wound moves, aiding in faster healing and preventing bacterial growth. Another breakthrough is antimicrobial photodynamic therapy (aPDT), which is proving effective for nasal decolonization, particularly in orthopedic and spinal surgeries, leading to fewer SSIs. These developments highlight a comprehensive approach to preventing SSIs, combining novel materials, targeted treatments, and improved infection control strategies for better patient recovery.
Recent Developments in Surgical Site Infections Market:
- In December 2024, PolyPid announced that the independent Data Safety Monitoring Board (DSMB) had reviewed unblinded efficacy data from the first 430 patients enrolled in the SHIELD II Phase 3 trial evaluating D-PLEX100 for the prevention of surgical site infections (SSIs) in patients undergoing abdominal colorectal surgery with large incisions. Based on its assessment, the DSMB recommended concluding the study upon reaching an enrollment of 800 patients, which is the lowest sample size reassessment stop after the initially planned minimum of 624 patients. At this interim analysis, the DSMB also had the option to recommend stopping the trial due to futility or overwhelming efficacy or to expand the sample size to a maximum of 1,100 patients. Additionally, the DSMB confirmed that D-PLEX100 has maintained a good safety profile in the SHIELD II trial.
- In October 2024, PolyPid announced the publication of a study in the International Journal of Surgery that highlights the results of the Phase 3 SHIELD I trial of D-PLEX100 for preventing surgical site infections in abdominal colorectal surgery, demonstrating its potential to enhance patient outcomes.
- In June 2024, Destiny Pharma announced that the UK Medicines and Healthcare Regulatory Agency (MHRA) granted an innovation passport to XF-73 nasal under the innovative licensing and access pathway (ILAP) for the prevention of post-surgical site infections.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the surgical site infections market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the surgical site infections market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current surgical site infections marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












