
ADME Toxicology Testing Market Size, Share, Trends and Forecast by Technology, Product Type, Method, Application, and Region, 2025-2033
ADME Toxicology Testing Market Size and Share:
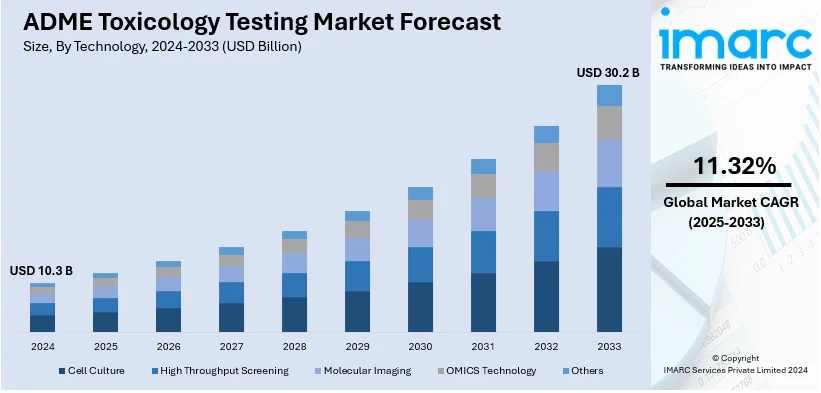
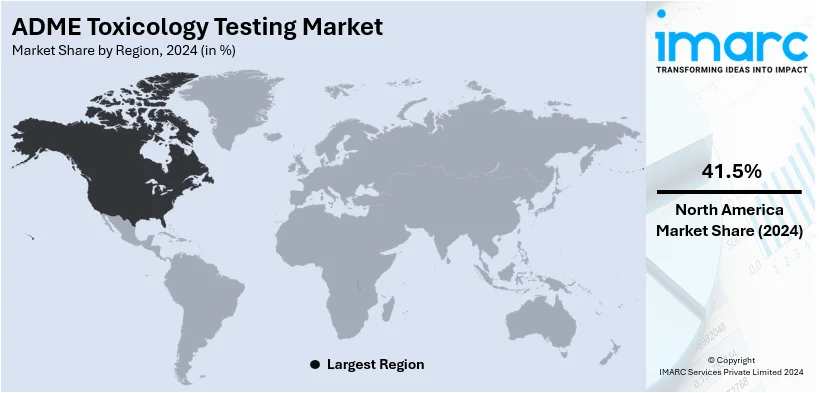
The global ADME toxicology testing market size reached USD 10.3 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 30.2 Billion by 2033, exhibiting a growth rate CAGR of 11.32% during 2025-2033. North America exhibits a clear dominance in the ADME toxicology testing market with a 41.5% share of the market. This region drives the ADME toxicology testing market through advanced healthcare infrastructure, high R&D investments, widespread adoption of predictive testing technologies, and regulatory emphasis on drug safety and efficacy standards.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 10.3 Billion |
|
Market Forecast in 2033
|
USD 30.2 Billion |
| Market Growth Rate 2025-2033 | 11.32% |
The growing importance of reducing post-discovery drug phase failures is a key driver for the ADME toxicology testing market. Pharmaceutical and biotechnology companies are increasingly focused on innovating modern ADME toxicology methods, which are essential for early assessment of absorption, distribution, metabolism, and excretion profiles, as well as toxicity risks. This ongoing advancement is contributing to the continued growth of the drug development sector. For example, in 2024, Beckman Coulter Life Sciences took the revolutionary step of bringing in the Cydem VT Automated Clone Screening System, a process that has the capacity of automatically developing a cell line with 90% fewer steps, thus making biological drug discovery a completely passive process. This approach minimizes costly setbacks during clinical trials, enhances drug safety profiles, and accelerates time-to-market for new therapies. Regulatory authorities' growing demand for stringent preclinical testing standards further bolsters adoption, positioning ADME toxicology testing as a critical component in modern drug discovery and development pipelines, driving significant market growth.
The United States is demonstrating magnifying growth in the ADME toxicology testing market due to its innovative pharmaceutical and biotechnology sectors, significant drug development research, and strict regulatory measures for the safety of drugs. For example, in 2024, Insilico Medicine presented five preclinical cancer drug programs at the 2024 AACR Annual Meeting. The company’s AI platform developed small-molecule inhibitors, including ISM6331 targeting TEAD and ISM8001, a dual FGFR2/FGFR3 inhibitor, which showed 80-120% tumor growth inhibition in preclinical models. Insilico also secured partnerships, including an $80M deal with Exelixis and a $500M agreement with Menarini for additional cancer treatments. Additionally, the integration of advanced technologies like high-throughput screening and in silico modeling, alongside growing collaborations between academia and industry, fuels the demand for precise toxicology testing solutions, reinforcing the U.S.'s leadership in ADME testing innovation.
ADME Toxicology Testing Market Trends:
Increasing Prevalence of Chronic Diseases
One of the principal factors driving growth in the ADME toxicology testing sector is the rise of chronic diseases worldwide. Serious ailments such as cancer, diabetes, and cardiovascular diseases need the need for lots of new medicines to be researched and developed. The World Health Organization says noncommunicable diseases (NCDs) cause 41 million deaths each year, making up 74% of all deaths globally. Of these, 17 million people die before age 70, with 86% of these early deaths happening in low- and middle-income countries. ADME toxicity testing stands out as the most important method of ensuring the safety and efficacy of the pharmaceuticals concerned. In the midst of the continued health problems and conditions caused by some diseases all over the world, there is a big need for the reduction of side effects of drugs as well as the safety of patients through drug testing. ADME toxicology testing provides crucial information on how drugs are absorbed, distributed, metabolized, and eliminated by the body. This process is essential for developing safe and effective treatments for chronic conditions. Thus, this continued demand for new pharmaceutical solutions to chronic conditions drives the growth of the market.
Technological Advancements
Technological advancements are rapidly increasing the ADME toxicology testing market. The modern testing processes like in vitro and in silico models over traditional methods of in vivo are increasingly adopted with a lot more accuracy, efficiency, and cost-effectiveness in the testing options. For instance, in 2024, Capstan Therapeutics secured $175 million to advance its in vivo cell therapy platform, which aims to reprogram cells within the body and move its lead candidate into clinical trials. This type of new approach is animal test-dependent to a reduced degree, complies with ethical considerations, and shows quicker results. Technological advances have also facilitated the creation of high-throughput screening methods, where it is possible to test multiple drug compounds at the same time, greatly speeding up the drug development process. In addition, the progress in computational biology and bioinformatics is improving the predictability of ADME toxicology tests, thus increasing their reliability and efficiency.
Increasing Regulatory Compliance
The strict regulatory measures made by authorities like the FDA and EMA for drug approval are another significant driver for the ADME Toxicology Testing market. These regulatory agencies require safety studies of novel drug substances to be conducted before the latter can be approved for human use. For example, in April 2024, the FDA approved Selarsdi 45 mg/0.5 ml and 90 mg/ml injections for subcutaneous administration to treat moderate to severe plaque psoriasis and active psoriatic arthritis in patients aged 6 and older. Thus, ADME toxicology testing is an important component since it provides necessary information on potential toxic effects on human health. In new compound development, new complexity emerges where people, in general, are much more sophisticated in drug-induced toxicities, and this brings stronger regulatory requirements.
Expanding Pharmaceutical Industry
A key driver of the ADME market is the substantial rise in research and development spending within the pharmaceutical industry. As companies allocate more resources to developing novel drugs, the demand for thorough ADME toxicology testing grows, ensuring both the safety and effectiveness of these medications. This testing is critical in identifying potential toxic effects and metabolic pathways early in the drug development process, thereby reducing the risk of late-stage failures. Increasing overall sales of pharmaceuticals across the globe coupled with rising demand for innovative drugs is further augmenting research and development. According to a recent analysis from one of the leading pharma consulting firms, the overall spending and global demand for medicines will rise over the next five years to around USD 1.9 Trillion by 2027. Additionally, the increasing complexity of emerging pharmaceutical compounds has led to a greater dependence on advanced ADME toxicology studies to comprehend how these drugs interact within the body. This demand is further amplified by stringent regulatory standards, which require comprehensive toxicology testing prior to drug approval, fueling growth in this sector.
ADME Toxicology Testing Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global ADME toxicology testing market, along with forecast at the global, regional, and country levels from 2025-2033. The market has been categorized based on technology, product type, method, and application.
Analysis by Technology:
- Cell Culture
- High Throughput Screening
- Molecular Imaging
- OMICS Technology
- Others
Cell culture leads the market with around 43.6% of the market share in 2024. This recognition is associated with the ability to mimic the behavior of living organisms, producing accurate and reliable data for drug absorption, distribution, metabolism, and excretion research. The application of sophisticated methods, like 3D cell cultures, and organ-on-a-chip models, has significantly increased the significance of cell culture in preclinical studies. Through the ability to decrease the use of animals, testing and to enhance predictive capability, cell culture remains a leader in the development of drugs.
Analysis by Product Type:
- Instruments
- Software Solutions
- Assay Systems
- Reagents
- Others
This leadership of software solutions is to a great extent due to their effectiveness in making data analysis more efficient and decision-making more effective in several different industries. Their usage is the most in the fields of healthcare, finance, and logistics for operations such as predictive modeling, automation, and operational optimization. Advanced software, including AI-driven platforms and cloud-based tools, enables organizations to process large datasets accurately and efficiently. The demand for software solutions is further fueled by the need for scalability, cost reduction, and enhanced productivity across diverse applications.
Analysis by Method:
- In-Vivo
- In-Vitro
- In-Silica
- Others
In-vivo leads the market as methods play a crucial role in making toxicology studies more capable and more accurate by giving more sophisticated methods of data analysis, modeling, and simulation. Through the alignment of software and cloud-based AI tools, reliable results of drug absorption, distribution, metabolism, and excretion are obtained, enabling faster and more cost-effective preclinical evaluations. Their integration reduces the reliance on manual processes and animal testing, streamlining drug development pipelines. The growing emphasis on precision and regulatory compliance further drives their adoption.
Analysis by Application:
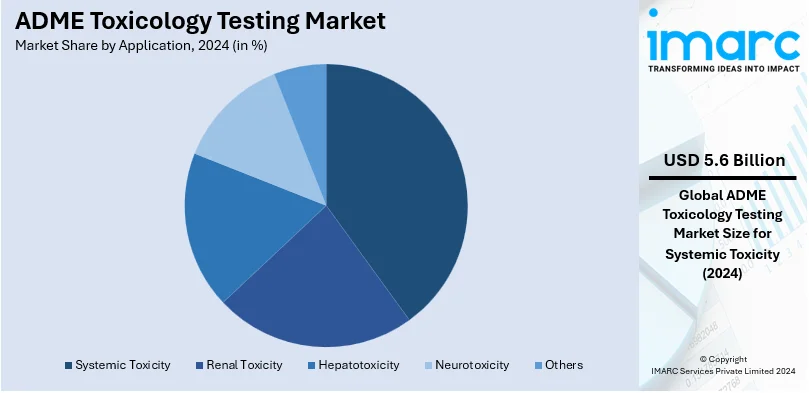
- Systemic Toxicity
- Renal Toxicity
- Hepatotoxicity
- Neurotoxicity
- Others
Systematic toxicity leads the market with around 53.8% of the market share in 2024. This testing focuses on assessing the adverse effects of drug candidates on various organ systems over time, ensuring comprehensive safety evaluations. It plays a critical role in identifying potential long-term and cumulative toxicities that could compromise drug efficacy or patient health. Regulatory bodies emphasize systematic toxicity studies to meet stringent safety standards. Advances in predictive modeling and integration with in-vivo and in-vitro techniques further enhance its precision, solidifying its market leadership.
Regional Analysis:
- North America
- United States
- Canada
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
In 2024, North America accounted for the largest market share of over 41.5%. This leadership is driven by the region's advanced healthcare infrastructure, significant investment in pharmaceutical and biotechnology research, and a strong focus on regulatory compliance. The widespread adoption of advanced technologies, such as high-throughput screening and in-silico modeling, enhances the precision and efficiency of toxicology testing. Additionally, the growing prevalence of chronic diseases and demand for innovative drug therapies further fuel market growth. North America remains at the forefront of innovations and industry advancements in ADME toxicology testing.
Key Regional Takeaways:
United States ADME Toxicology Testing Market Analysis
The US accounts for 84.8% share of the market in North America. The strong pharmaceutical and biotechnology industries in the US are driving the market for ADME toxicity testing. To guarantee drug safety and efficacy, dependable ADME (Absorption, Distribution, Metabolism, and Excretion) testing is essential due to significant investment in drug development and strict regulatory standards enforced by organizations such as the FDA. More than USD 100 Billion was spent on pharmaceutical R&D in the United States in 2022, which greatly increased the need for sophisticated testing techniques. Although this is a rather expanding market, around less than 30% of the newly discovered drugs successfully transition to Phase III clinical trials after passing through Phase II. Examples of technology revolutions that are saving costs and time in the testing procedures are high-throughput screening, as well as in silico testing. More dependence on artificial intelligence by humans in the ADME investigation satisfies the demand for techniques of non-animal testing in which better predictive toxicology becomes feasible. Another area that the U.S. is experiencing is an upsurge in outsourcing ADME services to dedicated CROs for greater efficiency and reduced cost-effectiveness. The development of chronic diseases and customized therapies also generates a high need for precise toxicological tests. For example, accurate profiles for ADME are a significant requirement for targeted anticancer therapy. Intersectoral agreements of the government with the private sectors and the academia—like NIH's NCATS program—is supporting the market to grow as well. The U.S. industry is likely to lead with a healthy compound annual growth rate (CAGR) propelled by ongoing innovation and regulatory compliance.
Europe ADME Toxicology Testing Market Analysis
Due to strict regulatory frameworks, including the European Medicines Agency's (EMA) recommendations for medication safety assessment, Europe is a major player in the ADME toxicity testing market. Key contributions include nations like Germany, the United Kingdom, and France, which profit from robust pharmaceutical industries and significant R&D expenditures. the pharmaceutical business made a substantial contribution to the EU economy in 2022, contributing Euro 311 Billion (USD 326 Billion). However, according to the most recent data from the European Federation of Pharmaceutical Industries and Associations (EFPIA), Europe's R&D investment growth has averaged 4.4% per year since 2010. The region's focus on minimizing animal testing is consistent with developments in substitute techniques like as computational toxicology and organ-on-a-chip technology. The need for thorough toxicological testing is further fuelled by programs like REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals), particularly for environmental safety. ADME testing is required due to the rising incidence of lifestyle-related diseases such as diabetes and cardiovascular problems, which drive medication development efforts. Furthermore, the necessity for accurate ADME profiling is increased by Europe's emphasis on biologics and personalised treatment. With a growing ecosystem of CROs and government support for innovative research, the market is projected to grow steadily in the coming years.
Asia Pacific ADME Toxicology Testing Market Analysis
The market for ADME toxicity testing is growing quickly in Asia-Pacific due to the expansion of pharmaceutical manufacturing in nations like China and India. Global pharmaceutical companies are drawn to the region's affordable drug development capabilities, which increases demand for ADME testing services. As Asia accounts for a growing share of their global revenues and, more significantly, most of their growth, many multinational pharmaceutical firms are witnessing this personally. As per several industrial reports, about 20% to 30% of their 2021 sales came from Asia, with China accounting for about half of that total. More than 220 novel treatments have been approved since China joined the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and the period between China's approval and the first approval has been reduced. Government programs like China's emphasis on developing breakthrough medicines and India's "Pharma Vision 2020" also help the market grow. Adoption of ADME testing is fuelled by the incidence of chronic diseases and rising healthcare costs, which increase the demand for safer medications. To satisfy the region's expanding needs, technological innovations including automated testing and high-throughput are being adopted. Asia-Pacific is expected to grow at a faster rate than the rest of the world, making it a major hub for ADME toxicity testing services.
Latin America ADME Toxicology Testing Market Analysis
The market for ADME toxicity testing is expanding in Latin America owing to increased pharmaceutical research and development as well as growing healthcare consciousness. Due in large part to expanding domestic drug production and government funding for research projects, Brazil, Mexico, and Argentina are major producers. Due to the region's emphasis on generic and biosimilar medications, significant ADME testing is required for regulatory clearance. Testing skills are further improved by collaborations between domestic and international pharmaceutical businesses. The need for safe and efficient drugs is also fuelled by the increase in chronic illnesses and easier access to healthcare systems. With its growing pharmaceutical infrastructure and affordable services, Latin America offers a profitable market. Moreover, in Latin America and the Caribbean, chronic conditions like heart disease, cancer, and diabetes are leading contributors to death and disability worldwide. Diabetes affects one in ten adults, while nearly 80% of fatalities are linked to these long-term illnesses, as per an industrial report.
Middle East and Africa ADME Toxicology Testing Market Analysis
The MENA region is experiencing a troubling increase in chronic conditions. Heart disease, diabetes, cancer, and respiratory disorders represent a substantial share of the region's overall health challenges. According to the World Health Organization (WHO), NCDs account for approximately 70% of deaths in the region. Urbanization, sedentary lifestyles, unhealthy diets, tobacco use, and obesity contribute to the high prevalence of these diseases. The increased pharmaceutical investments in nations like South Africa and the United Arab Emirates are driving growth in the Middle East and Africa (MEA) ADME toxicity testing market. The need for ADME services is increased by growing healthcare infrastructure and government funding for research and development. The necessity for accurate toxicological testing is fuelled by the region's rising clinical trials and high prevalence of infectious diseases. Partnerships with international pharmaceutical companies also improve local testing capacities. The MEA market is anticipated to increase steadily as knowledge of cutting-edge ADME technology rises, albeit more slowly than in other areas.
Competitive Landscape:
Market leaders are pursuing strategic actions to strengthen their market position. These efforts involve mergers and acquisitions to broaden their range of services, partnerships with pharmaceutical firms for early-stage toxicity evaluations, and the creation of advanced technologies for ADME testing. Additionally, market leaders are focusing on geographic expansion, especially in high-growth regions like Asia-Pacific and Latin America, to tap into emerging markets. For instance, in 2024, BioIVT showcased its new Ki67 tissue staining service for ADC development at Biomarkers U.S. 2024 and SITC 2024, emphasizing its commitment to advancing drug and diagnostic research.
The report provides a comprehensive analysis of the competitive landscape in the ADME toxicology testing market with detailed profiles of all major companies, including:
- Agilent Technologies Inc.
- Beckman Coulter Inc. (Danaher Corporation)
- Bioivt LLC
- Bio-Rad Laboratories Inc.
- Charles River Laboratories International Inc.
- Cyprotex Plc (Evotec AG)
- Molecular Discovery Ltd.
- Perkinelmer Inc.
- Promega Corporation
- Thermo Fisher Scientific, Inc.
Latest News and Developments:
- November 2024: An artificial intelligence (AI) algorithm has been created by researchers at the National Institutes of Health (NIH) to aid expedite the process of connecting prospective volunteers with pertinent clinical research trials that are published on ClinicalTrials.gov. According to a study in Nature Communications, the AI system TrialGPT was able to locate pertinent clinical trials for which a person qualifies and offer a synopsis that eloquently describes how the individual satisfies the requirements for study enrolment.
- March 2024: CN-Bio offers both standard and customized in vitro investigations in order to screen novel therapy candidates, investigate mechanisms of action, and speed up the design of clinical trials. Drug makers who wish to have instant access to organ-on-a-chip (OOC) technology without having to make equipment investments can benefit from their services.
- March 2023: Agilent Technologies' acquisition of e-MSion, the developer of ExD cell technology, represents a strategic move by Agilent to strengthen its position in the field of mass spectrometry and analytical chemistry. ExD cell technology is known for its capabilities in high-resolution mass spectrometry and ion mobility, making it a valuable addition to Agilent's portfolio. This acquisition aligns with Agilent's commitment to delivering innovative solutions to its customers in the life sciences and chemical analysis sectors.
- January 2020: Thermo Fisher Scientific's introduction of a next-generation, compressor-free plate sealer in the biotechnology and pharmaceutical sectors signifies a significant advancement in laboratory automation. This innovation streamlines plate sealing processes and also minimizes the need for operator maintenance, enhancing overall operational efficiency. The plate sealer's customization features cater to the diverse requirements of research laboratories, offering flexibility and adaptability for a range of applications. Its integration capabilities with robotic systems further boost productivity, making it a valuable tool for high-throughput environments. Thermo Fisher Scientific's dedication to creating cutting-edge solutions steps in line with the pursuit of reliability, precision, and user-friendliness in lab automation for research, drugdiscovery, or academic projects.
ADME Toxicology Testing Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Technologies Covered | Cell Culture, High Throughput Screening, Molecular Imaging, OMICS Technology, Others |
| Product Types Covered | Instruments, Software Solutions, Assay Systems, Reagents, Others |
| Methods Covered | In-Vivo, In-Vitro, In-Silica, Others |
| Applications Covered | Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Agilent Technologies Inc., Beckman Coulter Inc. (Danaher Corporation), Bioivt LLC, Bio-Rad Laboratories Inc., Charles River Laboratories International Inc., Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., Promega Corporation Thermo Fisher Scientific Inc. etc. (Please note that this is only a partial list of the key players, and the complete list is provided in the report.) |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the ADME toxicology testing market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the global ADME toxicology testing market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the ADME toxicology testing industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
Key Questions Answered in This Report
The ADME toxicology testing market was valued at USD 10.3 Billion in 2024.
The ADME toxicology testing market is projected to exhibit a CAGR of 11.32% during 2025-2033, reaching a value of USD 30.2 Billion by 2033.
The ADME toxicology testing market is driven by rapid growth of technology to predict test results, increasing R&D investments, priority of safety notes in the drug approval process, and the need to cut down on late-stage drug development failures. The growing number of chronic diseases and the demand for cheaper, faster, and more sensitive testing solutions are the key drivers of market growth.
North America currently dominates the market, accounting for a share of around 41.5%. The dominance is driven by advanced healthcare infrastructure, rising drug development activities, stringent regulatory requirements, increasing adoption of in vitro methods, and growing focus on precision medicine.
Some of the major players in the ADME toxicology testing market include Agilent Technologies Inc., Beckman Coulter Inc. (Danaher Corporation), Bioivt LLC, Bio-Rad Laboratories Inc., Charles River Laboratories International Inc., Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., and Promega Corporation Thermo Fisher Scientific Inc., among others.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












