
Allergy Relief Medication Manufacturing Plant Project Report 2026: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue
Report Overview:
IMARC Group’s report, titled “Allergy Relief Medication Manufacturing Plant Project Report 2026: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue” provides a complete roadmap for setting up an allergy relief medication manufacturing plant. It covers a comprehensive market overview to micro-level information such as unit operations involved, raw material requirements, utility requirements, infrastructure requirements, machinery and technology requirements, manpower requirements, packaging requirements, transportation requirements, etc. The allergy relief medication project report provides detailed insights into project economics, including capital investments, project funding, operating expenses, income and expenditure projections, fixed costs vs. variable costs, direct and indirect costs, expected ROI and net present value (NPV), profit and loss account, financial analysis, etc.
.webp)
allergy relief medication are designed to alleviate the symptoms caused by an overreaction of the immune system to harmless substances. They are available in the form of pills, nasal sprays, eye drops, or liquids and are formulated to target different allergens and symptoms. They are manufactured by reputable pharmaceutical companies and typically contain active ingredients that effectively counter the body's immune response to allergens. One of the primary advantages of allergy relief medication is its quick and reliable relief from bothersome symptoms like sneezing, itching, and congestion. They enable individuals to manage their allergies, thereby improving their overall quality of life. Furthermore, they are widely available over the counter (OTC), offering convenience and accessibility to consumers.
The global allergy relief medication market is influenced by the rising prevalence of allergies worldwide, triggered by environmental factors and lifestyle changes. Moreover, increasing awareness among the population about allergy symptoms and the availability of effective treatments are supporting the market growth. Additionally, advancements in medical research and technology contribute to the development of innovative and more efficient allergy relief medication, which is providing an impetus to the market growth. Besides this, the growing geriatric population and expanding urbanization and pollution levels are contributing to the escalating incidence of allergies, further propelling the market growth. Furthermore, supportive government policies and initiatives to improve healthcare accessibility and affordability is stimulating the market growth. Other factors, such as increased spending on healthcare and the expansion of healthcare facilities, are creating a conducive environment for market growth.
The following aspects have been covered in the report on setting up an allergy relief medication manufacturing plant:
- Market Analysis:
- Market Performance
- Market Breakup by Segment
- Market Breakup by Region
- Price Analysis
- Impact of COVID-19
- Market Outlook
The report provides insights into the landscape of the allergy relief medication industry at the global level. The report also provides a segment-wise and region-wise breakup of the global allergy relief medication industry. Additionally, it also provides the price analysis of feedstocks used in the manufacturing of allergy relief medication, along with the industry profit margins.
- Detailed Process Flow:
- Product Overview
- Unit Operations Involved
- Mass Balance and Raw Material Requirements
- Quality Assurance Criteria
- Technical Tests
The report also provides detailed information related to the process flow and various unit operations involved in an allergy relief medication manufacturing plant. Furthermore, information related to mass balance and raw material requirements has also been provided in the report with a list of necessary quality assurance criteria and technical tests.
- Project Details, Requirements and Costs Involved:
- Land, Location and Site Development
- Plant Layout
- Machinery Requirements and Costs
- Raw Material Requirements and Costs
- Packaging Requirements and Costs
- Transportation Requirements and Costs
- Utility Requirements and Costs
- Human Resource Requirements and Costs
The report provides a detailed location analysis covering insights into the land location, selection criteria, location significance, environmental impact, and expenditure for setting up an allergy relief medication manufacturing plant. Additionally, the report also provides information related to plant layout and factors influencing the same. Furthermore, other requirements and expenditures related to machinery, raw materials, packaging, transportation, utilities, and human resources have also been covered in the report.
Capital Expenditure (CapEx) and Operational Expenditure (OpEx) Analysis:
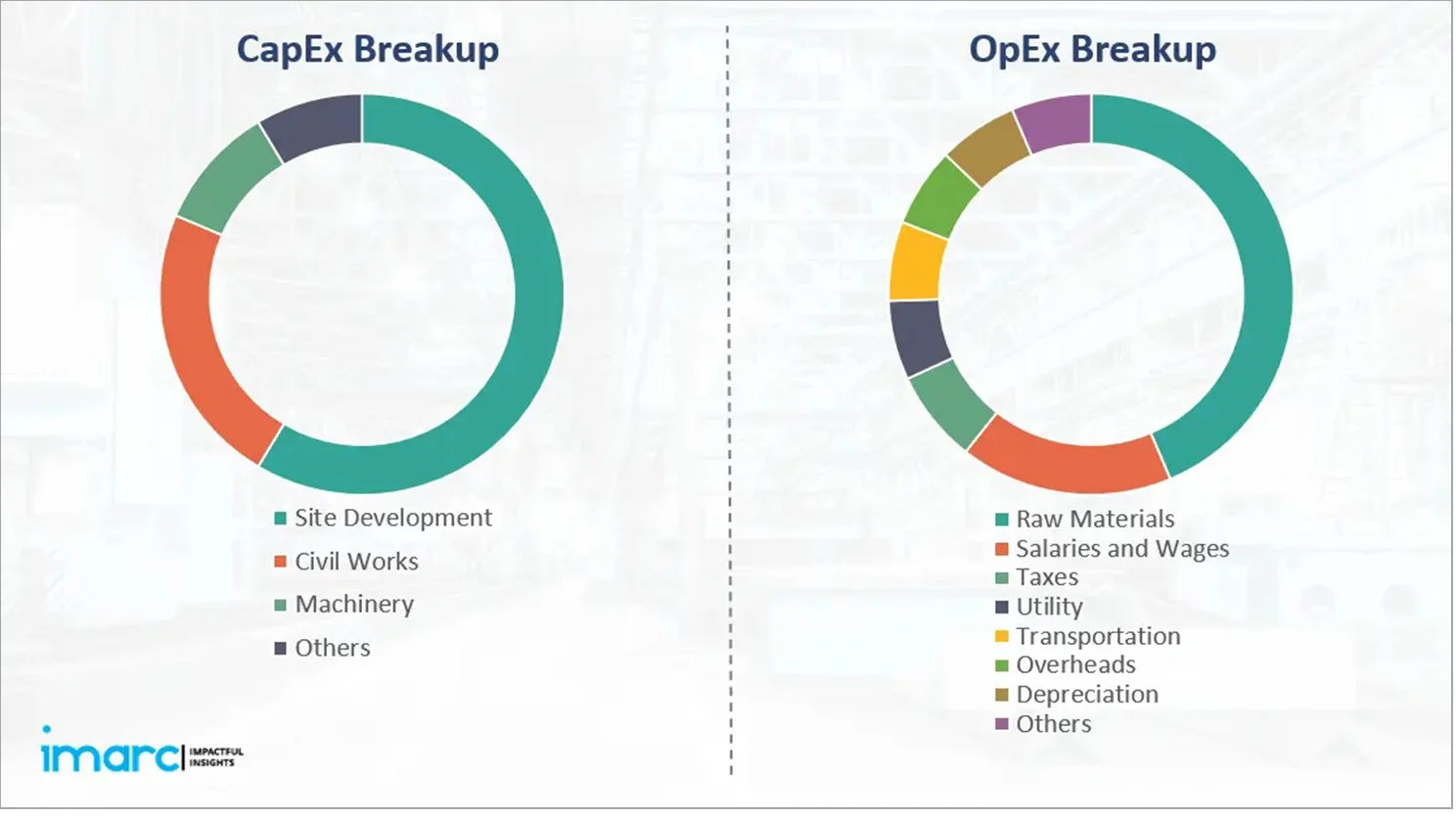
- Project Economics:
- Capital Investments
- Operating Costs
- Expenditure Projections
- Revenue Projections
- Taxation and Depreciation
- Profit Projections
- Financial Analysis
The report also covers a detailed analysis of the project economics for setting up an allergy relief medication manufacturing plant. This includes the analysis and detailed understanding of capital expenditure (CapEx), operating expenditure (OpEx), income projections, taxation, depreciation, liquidity analysis, profitability analysis, payback period, NPV, uncertainty analysis, and sensitivity analysis. Furthermore, the report also provides a detailed analysis of the regulatory procedures and approvals, information related to financial assistance, along with a comprehensive list of certifications required for setting up an allergy relief medication manufacturing plant.
Profitability Analysis:
| Particulars | Unit | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|---|
| Total Income | US$ | XX | XX | XX | XX | XX |
| Total Expenditure | US$ | XX | XX | XX | XX | XX |
| Gross Profit | US$ | XX | XX | XX | XX | XX |
| Gross Margin | % | XX | XX | XX | XX | XX |
| Net Profit | US$ | XX | XX | XX | XX | XX |
| Net Margin | % | XX | XX | XX | XX | XX |
Report Coverage:
| Report Features | Details |
|---|---|
| Product Name | Allergy Relief Medication |
| Report Coverage | Detailed Process Flow: Unit Operations Involved, Quality Assurance Criteria, Technical Tests, Mass Balance, and Raw Material Requirements Land, Location and Site Development: Selection Criteria and Significance, Location Analysis, Project Planning and Phasing of Development, Environmental Impact, Land Requirement and Costs Plant Layout: Importance and Essentials, Layout, Factors Influencing Layout Plant Machinery: Machinery Requirements, Machinery Costs, Machinery Suppliers (Provided on Request) Raw Materials: Raw Material Requirements, Raw Material Details and Procurement, Raw Material Costs, Raw Material Suppliers (Provided on Request) Packaging: Packaging Requirements, Packaging Material Details and Procurement, Packaging Costs, Packaging Material Suppliers (Provided on Request) Other Requirements and Costs: Transportation Requirements and Costs, Utility Requirements and Costs, Energy Requirements and Costs, Water Requirements and Costs, Human Resource Requirements and Costs Project Economics: Capital Costs, Techno-Economic Parameters, Income Projections, Expenditure Projections, Product Pricing and Margins, Taxation, Depreciation Financial Analysis: Liquidity Analysis, Profitability Analysis, Payback Period, Net Present Value, Internal Rate of Return, Profit and Loss Account, Uncertainty Analysis, Sensitivity Analysis, Economic Analysis Other Analysis Covered in The Report: Market Trends and Analysis, Market Segmentation, Market Breakup by Region, Price Trends, Competitive Landscape, Regulatory Landscape, Strategic Recommendations, Case Study of a Successful Venture |
| Currency | US$ (Data can also be provided in the local currency) |
| Customization Scope | The report can also be customized based on the requirement of the customer |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through email (We can also provide the editable version of the report in PPT/Word format on special request) |
Report Customization
While we have aimed to create an all-encompassing report, we acknowledge that individual stakeholders may have unique demands. Thus, we offer customized report options that cater to your specific requirements. Our consultants are available to discuss your business requirements, and we can tailor the report's scope accordingly. Some of the common customizations that we are frequently requested to make by our clients include:
- The report can be customized based on the location (country/region) of your plant.
- The plant’s capacity can be customized based on your requirements.
- Plant machinery and costs can be customized based on your requirements.
- Any additions to the current scope can also be provided based on your requirements.
Why Buy IMARC Reports?
- The insights provided in our reports enable stakeholders to make informed business decisions by assessing the feasibility of a business venture.
- Our extensive network of consultants, raw material suppliers, machinery suppliers and subject matter experts spans over 100+ countries across North America, Europe, Asia Pacific, South America, Africa, and the Middle East.
- Our cost modeling team can assist you in understanding the most complex materials. With domain experts across numerous categories, we can assist you in determining how sensitive each component of the cost model is and how it can affect the final cost and prices.
- We keep a constant track of land costs, construction costs, utility costs, and labor costs across 100+ countries and update them regularly.
- Our client base consists of over 3000 organizations, including prominent corporations, governments, and institutions, who rely on us as their trusted business partners. Our clientele varies from small and start-up businesses to Fortune 500 companies.
- Our strong in-house team of engineers, statisticians, modeling experts, chartered accountants, architects, etc. have played a crucial role in constructing, expanding, and optimizing sustainable manufacturing plants worldwide.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
Frequently Asked Questions
Capital requirements generally include land acquisition, construction, equipment procurement, installation, pre-operative expenses, and initial working capital. The total amount varies with capacity, technology, and location.
To start an allergy relief medication manufacturing business, one needs to conduct a market feasibility study, secure required licenses, arrange funding, select suitable land, procure equipment, recruit skilled labor, and establish a supply chain and distribution network.
Allergy relief medication manufacturing requires active pharmaceutical ingredients (APIs) such as antihistamines (e.g., cetirizine, loratadine, fexofenadine, diphenhydramine, or chlorpheniramine) depending on the formulation type. Excipients such as binders, disintegrants, fillers, stabilizers, and coating materials are also essential. Other raw materials include solvents, preservatives, and packaging materials such as blister films, bottles, and aluminum foils.
An allergy relief medication factory typically requires equipment such as granulators, blenders, tablet compression machines, capsule-filling units, coating pans, drying ovens, and liquid formulation mixers for syrups. Quality control instruments, sterilizers, filtration units, packaging lines (blister, bottle, or sachet), labeling machines, and cleanroom facilities with HVAC and dust extraction systems are also necessary to maintain GMP compliance.
The main steps generally include:
-
Procurement and testing of APIs and pharmaceutical-grade excipients to ensure purity and compliance with pharmacopeia standards
-
Weighing, blending, and granulation of raw materials to achieve uniform distribution of active and inactive ingredients
-
Drying and milling of granulated mixtures to obtain suitable particle size and flow properties
-
Compression or encapsulation of the prepared blend to form tablets or capsules based on the dosage form
-
Coating and polishing of tablets to improve stability, appearance, and swallowing comfort
-
Preparation of liquid formulations by dissolving or suspending active ingredients in suitable solvents with stabilizers and preservatives
-
Filling, sealing, and labeling of finished dosage forms using automated packaging lines
-
In-process and finished product testing for content uniformity, dissolution, and stability
-
Storage and distribution under controlled temperature and humidity conditions to maintain product efficacy and safety
Usually, the timeline can range from 18 to 36 months to start an allergy relief medication manufacturing plant, depending on factors like site development, machinery installation, environmental clearances, safety measures, and trial runs.
Challenges may include high capital requirements, securing regulatory approvals, ensuring raw material supply, competition, skilled manpower availability, and managing operational risks.
Typical requirements include business registration, environmental clearances, factory licenses, fire safety certifications, and industry-specific permits. Local/state/national regulations may apply depending on the location.
The top allergy relief medication manufacturers are:
-
GlaxoSmithKline plc (GSK)
-
Johnson & Johnson Services, Inc.
-
Sanofi S.A.
-
Bayer AG
-
Novartis AG
-
Haleon plc
-
Perrigo Company plc
-
Sun Pharmaceutical Industries Ltd.
Profitability depends on several factors including market demand, manufacturing efficiency, pricing strategy, raw material cost management, and operational scale. Profit margins usually improve with capacity expansion and increased capacity utilization rates.
Cost components typically include:
-
Land and Infrastructure
-
Machinery and Equipment
-
Building and Civil Construction
-
Utilities and Installation
-
Working Capital
Break even in an allergy relief medication manufacturing business typically range from 5 to 8 years, depending on scale, regulatory compliance costs, raw material pricing, and market demand. Efficient manufacturing and export opportunities can help accelerate returns.
Governments may offer incentives such as capital subsidies, tax exemptions, reduced utility tariffs, export benefits, or interest subsidies to promote manufacturing under various national or regional industrial policies.
Financing can be arranged through term loans, government-backed schemes, private equity, venture capital, equipment leasing, or strategic partnerships. Financial viability assessments help identify optimal funding routes.








 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization