
Australia Healthcare Cold Chain Logistics Market Size, Share, Trends and Forecast by Product, Segment, and Region, 2025-2033
Australia Healthcare Cold Chain Logistics Market Overview:
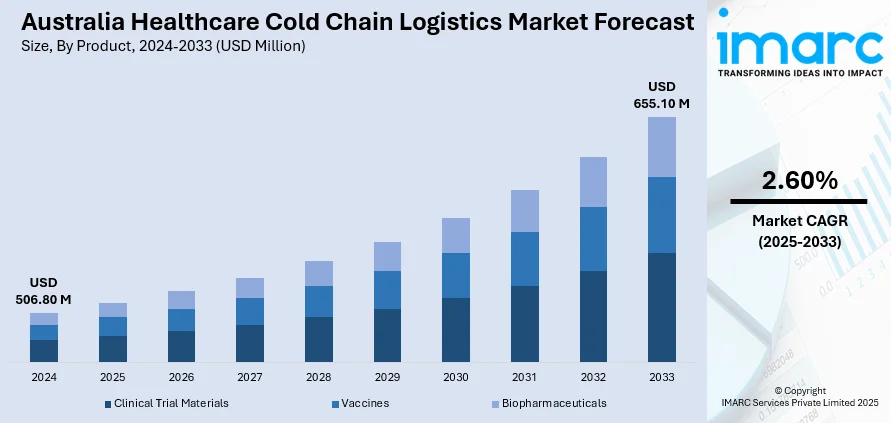
The Australia healthcare cold chain logistics market size reached USD 506.80 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 655.10 Million by 2033, exhibiting a growth rate (CAGR) of 2.60% during 2025-2033. The market is primarily being driven by stringent regulatory standards, the increasing demand for temperature-sensitive pharmaceuticals and vaccines, advancements in real-time monitoring technologies, and investments in specialized infrastructure to ensure product integrity and patient safety across the supply chain.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 506.80 Million |
| Market Forecast in 2033 | USD 655.10 Million |
| Market Growth Rate 2025-2033 | 2.60% |
Australia Healthcare Cold Chain Logistics Market Trends:
Increased Demand for Biopharmaceuticals and Vaccines
The growing demand for biopharmaceuticals and vaccines is a major driver of the healthcare cold chain logistics market in Australia. As biopharmaceutical products, such as monoclonal antibodies, gene therapies, and advanced vaccines, become more common, the need for strict temperature control during storage and transportation has increased. The COVID-19 pandemic underscored the critical need for reliable cold chain logistics, especially with the rollout of mRNA vaccines that require ultra-low temperature storage. The vaccine logistics market experienced an increase in demand post-pandemic, a trend expected to persist with the development of new vaccine technologies. As vaccine availability grows and biopharmaceutical production expands, this demand is set to continue, with Australia’s strategic position as a key player in the Asia-Pacific region further bolstering the cold chain logistics sector.
To get more information on this market, Request Sample
Technological Advancements in Real-Time Monitoring and Data Analytics
The Australia healthcare cold chain logistics market is expanding rapidly, owing to the implementation of new technologies such as real-time monitoring systems and data analytics to improve supply chain efficiency and dependability. Temperature-sensitive items are transported over great distances, and thus, maintaining constant temperatures is crucial for their efficacy and safety. As a result, tracking devices that can measure temperature, humidity, and position in real time have become increasingly common. These solutions provide logistics managers with quick notifications regarding any abnormalities, enabling timely, proactive actions to protect product integrity. With even tiny interruptions posing a danger to product quality and significant losses, IoT and blockchain technologies are gaining acceptance. These innovations enable tamper-proof data records and enhance transparency across the supply chain, improving operational efficiency by optimizing routes and minimizing delays.
Australia Healthcare Cold Chain Logistics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the region/country level for 2025-2033. Our report has categorized the market based on product and segment.
Product Insights:
- Clinical Trial Materials
- Vaccines
- Biopharmaceuticals
The report has provided a detailed breakup and analysis of the market based on the product. This includes clinical trial materials, vaccines, and biopharmaceuticals.
Segment Insights:
- Transportation
- Packaging
- Instrumentation
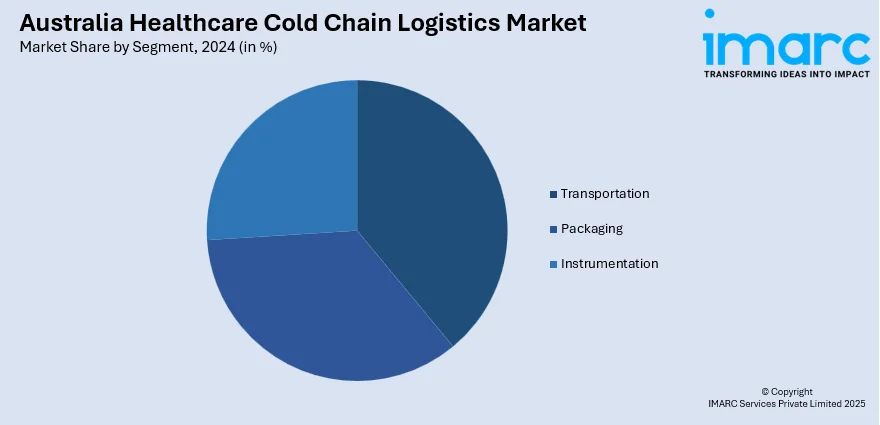
A detailed breakup and analysis of the market based on the segment have also been provided in the report. This includes transportation, packaging, and instrumentation.
Regional Insights:
- Australia Capital Territory & New South Wales
- Victoria & Tasmania
- Queensland
- Northern Territory & Southern Australia
- Western Australia
The report has also provided a comprehensive analysis of all the major regional markets, which include Australia Capital Territory & New South Wales, Victoria & Tasmania, Queensland, Northern Territory & Southern Australia, and Western Australia.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Australia Healthcare Cold Chain Logistics Market News:
- September 2024: Merck introduced greener alternatives for cold chain shipments in Australia, replacing 3.6 tons of non-recyclable expanded polystyrene (EPS) annually. To encourage a more sustainable value chain, the corporation will transition its Australian life science business to wool-pack insulation.
- December 2023: Sanofi Australia announced a partnership with supply chain provider Mediport to launch an active cold chain distribution service for medicines and vaccines in Tasmania. This service utilized vehicles equipped with dual temperature zones and advanced control systems that continuously monitor and report temperature fluctuations, ensuring enhanced protection of pharmaceutical products during transportation.
Australia Healthcare Cold Chain Logistics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered | Clinical Trial Materials, Vaccines, Biopharmaceuticals |
| Segments Covered | Transportation, Packaging, Instrumentation |
| Regions Covered | Australia Capital Territory & New South Wales, Victoria & Tasmania, Queensland, Northern Territory & Southern Australia, Western Australia |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Australia healthcare cold chain logistics market performed so far and how will it perform in the coming years?
- What is the breakup of the Australia healthcare cold chain logistics market on the basis of product?
- What is the breakup of the Australia healthcare cold chain logistics market on the basis of segment?
- What are the various stages in the value chain of the Australia healthcare cold chain logistics market?
- What are the key driving factors and challenges in the Australia healthcare cold chain logistics?
- What is the structure of the Australia healthcare cold chain logistics market and who are the key players?
- What is the degree of competition in the Australia healthcare cold chain logistics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Australia healthcare cold chain logistics market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Australia healthcare cold chain logistics market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Australia healthcare cold chain logistics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












