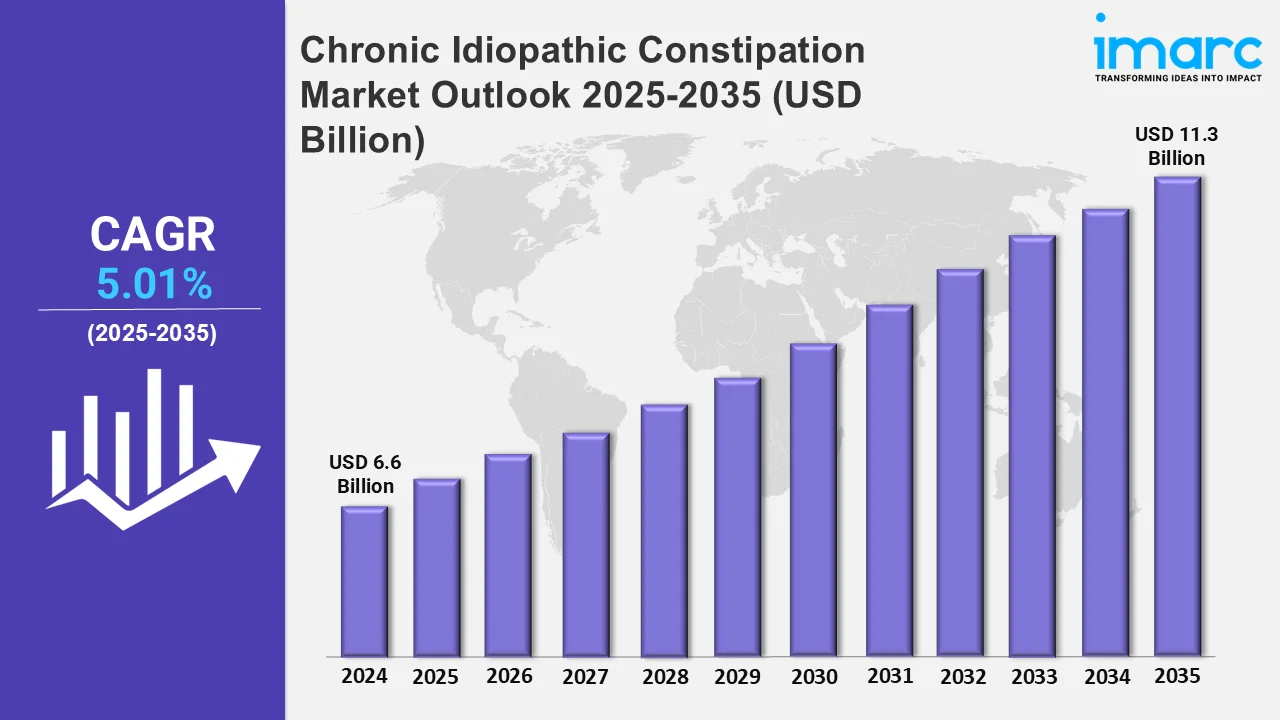
Chronic Idiopathic Constipation Market to Reach USD 11.3 Billion by 2035, Impelled by Heightened Rate of Risk Factors Contributing to Chronic Idiopathic Constipation
Chronic Idiopathic Constipation Market Outlook 2025-2035:
The 7 major chronic idiopathic constipation market reached a value of USD 6.6 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 11.3 Billion by 2035, exhibiting a growth rate (CAGR) of 5.01% during 2025-2035.
To get more information on this market, Request Sample
The market for chronic idiopathic constipation (CIC) is growing due to the rising prevalence of the condition, which stems from an aging population, sedentary lifestyles, and suboptimal dietary habits. In line with this, the advancement of innovative drugs and alternative treatments has captured the interest of patients and healthcare provider interests because of the availability of novel treatment options. The increased understanding of CIC's everyday life effects along with growing need for treatment solutions has driven up market demand. Moreover, market expansion is supported by both improved diagnostic technologies and growing expenditure in healthcare. The treatability of bowel disorders through prescription as well as over-the-counter treatments enhances accessibility, while the ongoing digestive health interest guarantees the CIC market's expansion.
Heightened Rate of Risk Factors Contributing to Chronic Idiopathic Constipation: Driving the Chronic Idiopathic Constipation Market
As our understanding of the condition grows, the demand for specialized treatments and diagnostic tools is also rising, driving the shift towards precision medicine. With the increase in lifestyle-related risk factors and the rising demand for better treatment options, the CIC market is expected to continue expanding in the future.
Chronic idiopathic constipation (CIC) market growth occurs due to maintaining factors associated with its increasing prevalence across populations. An aging population combined with sedentary life choices, unhealthy diet, and increasing stress levels drives the higher incidence of gastrointestinal problems leading to CIC. Older adults face a higher risk of chronic constipation due to age-related changes in digestion and the impact of medications, which often exacerbate bowel movement challenges. Moreover, the increasing understanding of CIC drives greater patient need for effective treatment methods, thereby favoring the market growth. In confluence with this, the growing importance of alternative medical treatments, including anti-diarrheal drugs and medications, which affect both guanylate cyclase-C agonists and serotonin receptors, persists along with traditional intervention methods like physical exercise advising and nutritional adjustments. Additionally, the development of innovative therapies targeting the microbiome and bio-based solutions is opening up new opportunities for the market. Improvements in healthcare access, particularly in emerging markets, are making it easier for more people to seek treatment for CIC, furthering market expansion.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The treatment options for chronic idiopathic constipation (CIC) are rapidly evolving, driven by the development of innovative therapies. Research has shown that traditional treatments consisting of fiber supplements and laxatives frequently fail at treating symptoms persistently, which generates substantial market need for new effective treatments. The pharmaceutical industry made substantial progress by developing medication which addresses CIC's fundamental causes including slow gut motility together with decreased fluid secretion and misbalanced gut microbiome. New medication categories with prokinetic agents, secretagogues, and neuromodulators are emerging to address varying aspects of the condition. Moreover, plecanatide and other new drugs act in the gut by enhancing chloride secretion which results in improved bowel movements and decreased abdominal distress. Other treatments, like target serotonin receptors to enhance gut motility, offering options for patients who don’t respond to conventional therapies. The market for CIC treatments is escalating as more medical professionals recognize the condition as a chronic issue that requires ongoing management. Pharmaceutical companies are investing heavily in the development of new drugs that offer fewer side effects and better adherence, which is helping to grow the market. Regulatory approvals for new therapies, as well as advancements in diagnostic techniques, are making treatments more accessible to patients. In addition to oral medications, injectable and biologic therapies are also being explored, offering more treatment options. As awareness of CIC increases and new treatments become available, the demand for effective solutions is expected to grow, driving further market expansion.
Marketed Therapies in Chronic Idiopathic Constipation Market
Linzess (Linaclotide): AbbVie/Ironwood Pharmaceuticals
Linzess (Linaclotide), developed by Ironwood Pharmaceuticals and AbbVie, is an oral medication approved for the treatment of chronic idiopathic constipation (CIC) in adults. It works as a guanylate cyclase-C agonist, increasing fluid secretion and accelerating bowel movements while reducing abdominal discomfort.
Motegrity (Prucalopride): Takeda
Motegrity (Prucalopride), developed by Takeda, is an oral medication approved for the treatment of chronic idiopathic constipation (CIC) in adults. It is a selective serotonin-4 (5-HT4) receptor agonist that enhances colonic motility, promoting bowel movements and alleviating constipation symptoms. With a well-established safety profile, it is designed to improve overall bowel function and reduce the discomfort associated with CIC.
Trulance (Plecanatide): Bausch Health Companies
Trulance (Plecanatide), developed by Bausch Health Companies, is an oral medication approved for the treatment of chronic idiopathic constipation (CIC) in adults. It functions as a uroguanylin analog, activating guanylate cyclase-C receptors in the gastrointestinal tract to increase fluid secretion and support regular bowel movements. Trulance is designed to provide effective relief from constipation while minimizing side effects such as diarrhea.
Amitiza (Lubiprostone): Mallinckrodt
Amitiza (Lubiprostone), developed by Mallinckrodt, is an oral medication approved for the treatment of chronic idiopathic constipation (CIC) in adults. It works by activating chloride channels in the intestinal lining, increasing fluid secretion to soften stool and promote regular bowel movements. Amitiza also helps alleviate associated symptoms such as bloating and abdominal discomfort, offering a well-tolerated treatment option for CIC.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Linzess (Linaclotide) | AbbVie/Ironwood Pharmaceuticals | Enterotoxin receptor agonists | Oral |
| Motegrity (Prucalopride) | Takeda | Serotonin 4 receptor agonists | Oral |
| Trulance (Plecanatide) | Bausch Health Companies | Enterotoxin receptor agonists | Oral |
| Amitiza (Lubiprostone) | Mallinckrodt | Chloride channel agonists | Oral |
Detailed list of marketed and emerging therapies in Chronic Idiopathic Constipation is provided in the final report…
Leading Companies in the Chronic Idiopathic Constipation Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global chronic idiopathic constipation market, several leading companies are at the forefront of developing integrated platforms to enhance the management of chronic idiopathic constipation. Some of the major players include Bausch Health Companies, Mallinckrodt, Takeda, AbbVie, and others. These companies are driving innovation in the chronic idiopathic constipation market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for chronic idiopathic constipation.
In early January 2025, ANI Pharmaceuticals announced the FDA approval and launch of Prucalopride Tablets, granted a 180-day Competitive Generic Therapy (CGT) exclusivity. Prucalopride is a selective serotonin-4 (5-HT4) receptor agonist that enhances colonic motility, offering a treatment option for adults with chronic idiopathic constipation.
Key Players in Chronic Idiopathic Constipation Market:
The key players in the chronic idiopathic constipation market who are in different phases of developing different therapies are Ironwood Pharmaceuticals, Takeda, ANI Pharmaceuticals, Sebela Pharmaceuticals, Bausch Health Companies, Mallinckrodt, AbbVie, and Others.
.webp)
Regional Analysis:
The major markets for chronic idiopathic constipation include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for chronic idiopathic constipation while also representing the biggest market for its treatment. Recent advancements in the treatment of chronic idiopathic constipation (CIC) include the development of new pharmacological agents such as plecanatide which improve bowel movements by targeting intestinal secretory pathways. Innovations like peripherally acting mu-opioid receptor antagonists address opioid-induced constipation. Digital health tools, such as biofeedback therapy and personalized apps, are enhancing patient self-management. Additionally, dietary and microbiome-based interventions, including prebiotics and probiotics, are gaining traction as complementary approaches. These advancements aim to improve symptom relief and quality of life for CIC patients.
Recent Developments in Chronic Idiopathic Constipation Market:
- In May 2023, the American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) released new guidelines for the pharmacological treatment of chronic idiopathic constipation (CIC) in adults. These guidelines are the first to recommend Magnesium Oxide and Senna as evidence-based treatment options. This joint clinical practice guideline, published today in the scientific journals of both societies, marks a significant step in advancing the management of chronic constipation.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the chronic idiopathic constipation market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the chronic idiopathic constipation market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current chronic idiopathic constipation marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












