
D-dimer Testing Market Report by Test Type (Clinical Laboratory Tests, Point-of-Care Tests), Product (Analyzers, Reagents and Consumables), Method (Enzyme-linked Immunosorbent Assay (ELISA), Latex-enhanced Immunoturbidimetric Assays, Fluorescence Immunoassays, and Others), Application (Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), and Others), End Use (Hospitals, Academic and Research Institutes, Diagnostic Centers, and Others), and Region 2026-2034
D-dimer Testing Market Size:
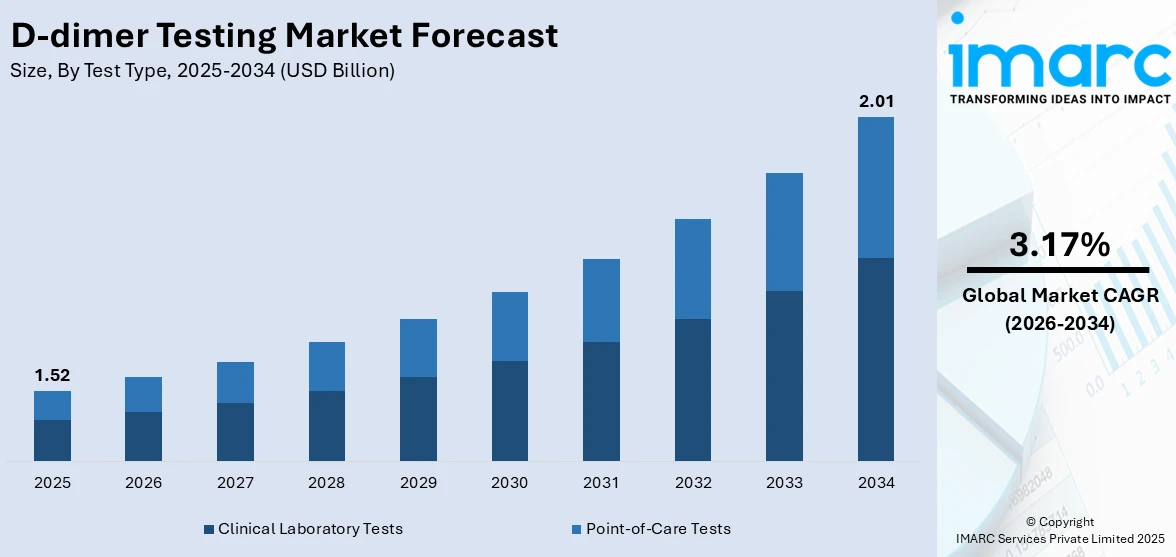
The global D-dimer testing market size reached USD 1.52 Billion in 2025. Looking forward, IMARC Group expects the market to reach USD 2.01 Billion by 2034, exhibiting a growth rate (CAGR) of 3.17% during 2026-2034. The market is experiencing steady growth driven by the rising prevalence of thrombotic diseases such as deep vein thrombosis and pulmonary embolism, particularly in aging populations, the increasing global healthcare expenditure across the globe, and continuous technological advancements in diagnostic technologies.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025
|
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
|
Market Size in 2025
|
USD 1.52 Billion |
|
Market Forecast in 2034
|
USD 2.01 Billion |
| Market Growth Rate 2026-2034 | 3.17% |
D-dimer Testing Market Analysis:
- Market Growth and Size: The market is experiencing robust growth, driven primarily by the rising prevalence of cardiovascular and thrombotic diseases. This rise in demand, coupled with increasing healthcare expenditure and insurance coverage, is expanding the market size, making it a vital segment in the diagnostic testing industry.
- Technological Advancements: Advancements in diagnostic technologies, including automation and AI integration, are revolutionizing the testing. These innovations lead to higher accuracy, efficiency, and user-friendliness, enhancing their adoption in various healthcare settings and contributing positively to market growth.
- Industry Applications: D-dimer tests are extensively used in diagnosing conditions such as deep vein thrombosis, pulmonary embolism, and stroke, particularly in aging populations. Their increasing use in preventive care and routine health check-ups signifies their growing importance in the healthcare industry.
- Key Market Trends: A notable trend in the market is the shift towards automated testing procedures, which offer more reliable and quicker results. There is also a growing focus on developing tests with higher specificity to reduce false positives, reflecting an emphasis on quality patient care.
- Geographical Trends: Geographically, developed regions including North America and Europe dominate the market due to advanced healthcare infrastructure, high healthcare spending, and awareness about thrombotic diseases. However, emerging economies in Asia-Pacific are rapidly catching up, driven by improving healthcare facilities and increasing prevalence of cardiovascular diseases.
- Competitive Landscape: The market is marked by the presence of several key players who are focusing on technological innovations and strategic partnerships to expand their market presence. This competitive environment fosters continual advancements in test accuracy and efficiency.
- Challenges and Opportunities: While the market faces challenges such as the need for highly skilled personnel to interpret test results, it also presents opportunities for developing simpler, more accessible testing methods. The expanding healthcare infrastructure in developing countries offers a significant opportunity for market expansion.
To get more information on this market Request Sample
D-dimer Testing Market Trends:
Rising prevalence of cardiovascular and thrombotic diseases
One of the primary factors driving the growth of the global market is the increasing prevalence of cardiovascular diseases (CVDs) and thrombotic disorders. This trend is especially pronounced in aging populations worldwide, where the risk of diseases such as deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke is significantly higher. Additionally, they are critical in the diagnosis and exclusion of these conditions. Along with this, the growing awareness about the importance of early detection and treatment of thrombotic disorders further provides a boost to the demand for these tests. As healthcare systems globally focus more on preventive care and early diagnosis, the demand is rising, thereby fueling market growth.
Advancements in diagnostic technologies
Technological advancements in diagnostic testing methods are significantly impacting the market. Modern D-dimer tests offer higher accuracy, quicker results, and are more user-friendly, which increases their adoption in both hospital and outpatient settings. In confluence with this, improved sensitivity and specificity of these tests reduce the likelihood of false positives and negatives, enhancing patient care quality. Therefore, this is positively influencing the market. Moreover, the integration of automation and AI in diagnostic procedures further streamlines the testing process, making it more efficient and reliable. Such technological innovations improve patient outcomes and drive the expansion and acceptance in various healthcare settings, contributing to market growth.
Increasing healthcare expenditure and insurance coverage
The global increase in healthcare expenditure and the expansion of insurance coverage are key factors contributing to the growth of the market. With more resources allocated to healthcare, there is a greater focus on comprehensive diagnostic testing, which includes D-dimer tests. In addition to this, the expansion of health insurance coverage in many countries is making these tests more accessible to a broader segment of the population. This increased accessibility encourages regular health check-ups and preventive testing, which in turn drives the demand. Furthermore, the overall improvement in healthcare infrastructure, particularly in developing countries, also plays a crucial role in the market's expansion.
D-dimer Testing Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the global, regional, and country levels for 2026-2034. Our report has categorized the market based on test type, product, method, application, and end use.
Breakup by Test Type:
- Clinical Laboratory Tests
- Point-of-Care Tests
Clinical laboratory tests account for the majority of the market share
The report has provided a detailed breakup and analysis of the market based on the test type. This includes clinical laboratory tests and point-of-care tests. According to the report, clinical laboratory tests represented the largest segment.
Clinical laboratory tests form the largest segment in the market. This dominance can be attributed to the high accuracy and reliability of these tests, which are crucial in diagnosing conditions like deep vein thrombosis and pulmonary embolism. Performed in well-equipped labs, these tests offer comprehensive analysis and are preferred in cases requiring detailed diagnostics. Additionally, the segment's growth is supported by the widespread availability of advanced healthcare infrastructure, particularly in developed countries, and the increasing prevalence of cardiovascular and thrombotic diseases.
On the contrary, the Point-of-Care (POC) tests segment, though smaller in comparison to clinical laboratory tests, is gaining traction in the market. These tests are designed for convenience and speed, providing results at the location of patient care, such as bedside in hospitals or in outpatient clinics. POC tests are particularly beneficial in urgent care scenarios where quick decision-making is crucial. Along with this, the rising demand for rapid and user-friendly testing options, along with advancements in technology making POC tests more accurate, is contributing to this segment's growth.
Breakup by Product:
- Analyzers
- Reagents and Consumables
Reagents and consumables hold the largest share of the industry
A detailed breakup and analysis of the market based on the product have also been provided in the report. This includes analyzers, and reagents and consumables. According to the report, reagents and consumables accounted for the largest market share.
The segment of reagents and consumables holds the largest share of the market. This segment's prominence is driven by the recurring need for these products in procedures. Reagents and consumables are essential for the accuracy and efficacy of the tests, and their demand is directly linked to the frequency of the testing. The continuous development of more specific and sensitive reagents further fuels the segment's growth. Additionally, as the prevalence of thrombotic diseases increases globally, the need for regular and widespread testing escalates, consequently enhancing the demand for these consumables.
On the other hand, the analyzers segment, while smaller compared to reagents and consumables, plays a crucial role in the market. These devices, ranging from basic to highly advanced systems, are essential for conducting D-dimer tests. The growth in this segment is primarily driven by technological advancements that lead to more sophisticated, accurate, and user-friendly analyzers. In addition, the development of automated systems that reduce manual intervention and the potential for error is particularly significant. While the high cost of advanced analyzers might be a limiting factor, especially in developing economies, the overall market trend towards automation and efficiency in diagnostics is likely to support the growth of this segment. Moreover, the increasing adoption in various healthcare settings further contributes to the demand for analyzers.
Breakup by Method:
- Enzyme-linked Immunosorbent Assay (ELISA)
- Latex-enhanced Immunoturbidimetric Assays
- Fluorescence Immunoassays
- Others
Enzyme-linked immunosorbent assay (ELISA) represents the leading market segment
The report has provided a detailed breakup and analysis of the market based on the method. This includes enzyme-linked immunosorbent assay (ELISA), latex-enhanced immunoturbidimetric assays, fluorescence immunoassays, and others. According to the report, enzyme-linked immunosorbent assay (ELISA) represented the largest segment.
The Enzyme-linked Immunosorbent Assay (ELISA) segment is a significant part of the market. ELISA is renowned for its high sensitivity and specificity, making it a preferred choice for accurately detecting D-dimer levels. This method involves an enzymatic reaction that produces a measurable signal, typically a color change, indicating the presence of D-dimer. Its widespread use in clinical laboratories stems from its reliability and the detailed information it provides, which is crucial for diagnosing thrombotic disorders.
Along with this, Latex-enhanced immunoturbidimetric assays are an important part of the market. This method involves agglutination of latex particles, which is measured turbidimetrically to determine the presence and concentration of D-dimer in the blood sample. The key advantage of this method is its speed and suitability for emergency settings where rapid results are essential. It is less sensitive than ELISA but offers a good balance between speed, ease of use, and accuracy, making it suitable for initial screening tests. The need for faster diagnostic methods in critical care and emergency medicine drives the demand for latex-enhanced immunoturbidimetric assays.
Apart from this, fluorescence Immunoassays represent a growing segment in the market. This method employs fluorescent labels, which offer high sensitivity and the ability to detect low levels of D-dimer. Fluorescence immunoassays are particularly valuable in settings where precise quantification of D-dimer is necessary. The advancement in fluorescence technology is leading to the development of more compact and user-friendly devices, increasing their adoption in various clinical settings. While this method is generally more expensive than others, its high sensitivity and specificity make it a valuable tool in the diagnosis and management of thrombotic disorders.
Breakup by Application:
Access the comprehensive market breakdown Request Sample
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism (PE)
- Disseminated Intravascular Coagulation (DIC)
- Others
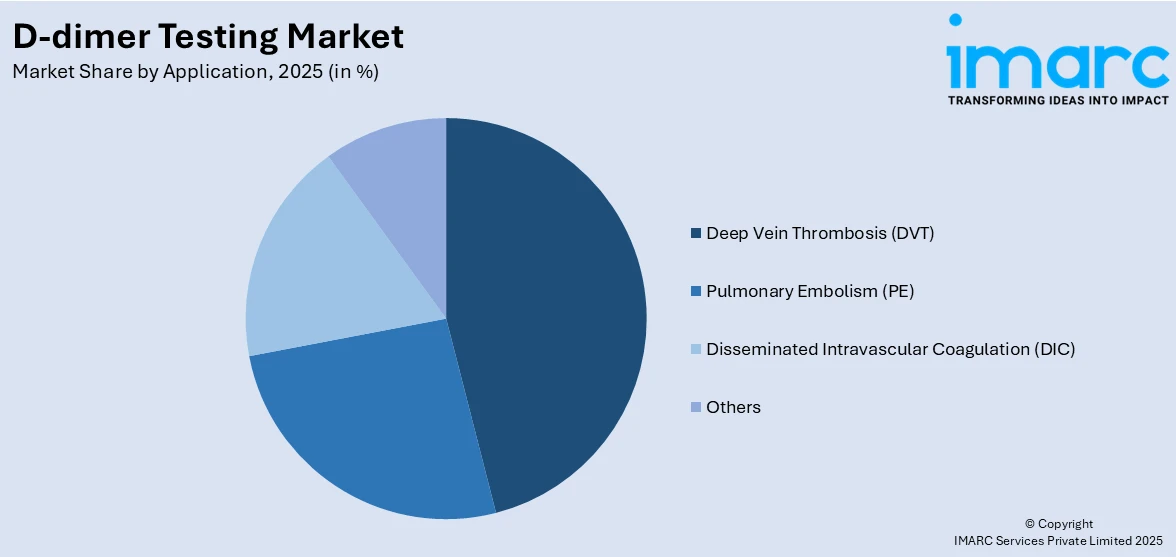
Deep vein thrombosis (DVT) exhibits a clear dominance in the market
A detailed breakup and analysis of the market based on the application have also been provided in the report. This includes deep vein thrombosis (DVT), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), and others. According to the report, deep vein thrombosis (DVT) accounted for the largest market share.
The deep vein thrombosis (DVT) segment is a major application area in the market. DVT is a condition characterized by the formation of blood clots in deep veins, usually in the legs. D-dimer tests are extensively used to exclude the diagnosis of DVT, especially in patients showing symptoms but having a low clinical probability of the condition. Additionally, the high demand for DVT diagnosis is attributed to its non-invasive nature and the ability to quickly rule out the condition, which is crucial in guiding further diagnostic or therapeutic actions. The rising incidence of DVT, partly due to increasing sedentary lifestyles and aging populations, is expected to continue driving the growth of this segment.
Apart from this, Pulmonary Embolism (PE) is another key application segment in the market. PE occurs when a blood clot, often originating from a DVT, lodges in the lungs, a condition that can be life-threatening. D-dimer tests are vital in the initial screening for PE, as elevated levels can indicate the presence of a clot. While a negative D-dimer test can help exclude PE in patients with low clinical probability, a positive result typically leads to further imaging tests. Along with this, the increasing awareness of PE symptoms and the critical need for prompt diagnosis to prevent fatalities are factors contributing to the growth of this segment in the market.
In addition, the disseminated intravascular coagulation (DIC) segment, though smaller compared to DVT and PE, is significant in the market. DIC is a complex condition characterized by the simultaneous occurrence of blood clotting and bleeding. It can be secondary to various medical conditions, including infections, cancer, and trauma. D-dimer tests are crucial in the diagnosis and management of DIC, as they help in identifying abnormal clot formation. The role in DIC is challenging due to the complexity of the condition but remains essential for its diagnostic value. The segment’s growth is influenced by the need for effective diagnostic tools in managing DIC, especially in critical care settings.
Breakup by End Use:
- Hospitals
- Academic and Research Institutes
- Diagnostic Centers
- Others
Hospitals dominate the market
The report has provided a detailed breakup and analysis of the market based on the end use. This includes hospitals, academic and research institutes, diagnostic centers, and others. According to the report, hospitals represented the largest segment.
The hospitals segment is the largest in the market. Hospitals are primary centers for the diagnosis and treatment of various thrombotic disorders such as deep vein thrombosis, pulmonary embolism, and disseminated intravascular coagulation, where D-dimer tests are frequently utilized. The high volume of patient traffic, coupled with the availability of comprehensive diagnostic facilities, contributes to the dominant share of this segment. Moreover, hospitals are often equipped with advanced testing equipment and skilled personnel, which is essential for accurate testing. Additionally, the increasing prevalence of cardiovascular and thrombotic diseases, alongside the growing emphasis on preventive healthcare and early diagnosis, further solidifies the hospitals segment as a key end-user in the market.
On the other hand, academic and research institutes form a vital segment of the market. This segment primarily focuses on research and development activities related to thrombotic disorders and the improvement of diagnostic techniques, including D-dimer tests. Academic institutions often collaborate with healthcare providers and industry players to conduct clinical trials and develop new testing methodologies. The contribution of this segment is crucial for advancing the understanding of thrombotic diseases and enhancing the accuracy, efficiency, and accessibility of D-dimer tests.
In addition, diagnostic centers are an important segment of the market. These centers specialize in providing a wide range of diagnostic services, including D-dimer tests, to patients referred by physicians or those seeking independent health check-ups. The convenience, accessibility, and often quicker turnaround times for test results make diagnostic centers a preferred choice for many patients. With the growing awareness of thrombotic disorders and the emphasis on routine health monitoring, the demand for these centers is increasing. The expansion of diagnostic centers, especially in urban areas and emerging economies, is enhancing their share in the market.
Breakup by Region:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
North America leads the market, accounting for the largest D-dimer testing market share
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share.
North America represents the largest segment of the market. This dominance is primarily attributed to the region's advanced healthcare infrastructure, high healthcare expenditure, and widespread awareness of thrombotic disorders. The United States plays a key role in this market, with a well-established network of healthcare facilities and a significant focus on research and development in medical technologies. Additionally, the presence of major pharmaceutical and biotechnology companies in this region, coupled with favorable government policies, further contributes to the robust growth of the market in North America.
Along with this, the Asia Pacific region is a rapidly growing segment of the market. This growth is driven by the increasing prevalence of cardiovascular diseases, improving healthcare infrastructure, and rising healthcare awareness among the population. Countries such as China and India, with their large populations and changing healthcare systems, are key contributors to the market's expansion in this region. Moreover, increasing investment in healthcare by both government and private sectors is expected to further propel the growth of the market in the Asia Pacific region.
In addition, Europe holds a significant position in the global market, characterized by advanced healthcare systems and a high prevalence of thrombotic diseases. The region's focus on preventive healthcare and early diagnosis, along with substantial healthcare spending, supports the demand for D-dimer tests. Countries including Germany, the UK, and France are leading contributors in this market, supported by their strong healthcare infrastructure and ongoing research in medical diagnostics.
Apart from this, the Latin American market is growing, although at a slower pace compared to North America and Europe. Factors such as improving healthcare infrastructure, increasing awareness of thrombotic conditions, and gradual economic development are driving the growth in this region. Countries such as Brazil and Mexico are leading this growth, though the market faces challenges such as limited access to advanced diagnostic technologies and a lack of uniform healthcare policies across different countries.
Moreover, the Middle East and Africa region constitute a smaller segment of the global market, but it is gradually growing. This growth is fueled by increasing healthcare investments in countries like Saudi Arabia and the United Arab Emirates. Concurrently, the rising focus on improving healthcare services and the increasing prevalence of lifestyle-related diseases are factors that could contribute to the growth of the market in this region.
Leading Key Players in the D-dimer Testing Industry:
The key players in the market are actively engaged in various strategic activities to strengthen their market position. These include research and development efforts to enhance the accuracy and efficiency of D-dimer tests, focusing on technological advancements such as automation and AI integration. They are also forming strategic partnerships and collaborations with healthcare institutions and other companies for technological exchange and market expansion. Moreover, numerous players are investing in expanding their geographical presence, particularly in emerging economies with growing healthcare sectors. Efforts in marketing and educational initiatives to increase awareness about thrombotic disorders and the importance of early diagnosis are also a significant part of their strategies. These comprehensive efforts by market leaders are aimed at maintaining their dominance and driving overall market growth and innovation.
The market research report has provided a comprehensive analysis of the competitive landscape. Detailed profiles of all major companies have also been provided. Some of the key players in the market include:
- Abbott Laboratories
- BioMedica Diagnostics
- biomérieux SA
- Diazyme Laboratories Inc. (General Atomics)
- F. Hoffmann-La Roche Ltd.
- HORIBA Ltd.
- Quidel Corporation
- Sekisui Diagnostics LLC (Sekisui Chemical Co. Ltd.)
- Siemens Healthcare GmbH
- Thermo Fisher Scientific Inc.
- Unbound Medicine Inc
- Werfen
(Please note that this is only a partial list of the key players, and the complete list is provided in the report.)
Latest News:
- October 18, 2023: Abbott Laboratories launched its ground-breaking vascular imaging platform with Ultreon 1.0 software in India.
- February 07, 2023: Diazyme Laboratories Inc. (General Atomics) obtained a December 6, 2022, Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration to utilize the Diazyme SARS-CoV-2 Neutralising Antibody CLIA Kit.
- September 8, 2022: F. Hoffmann-La Roche Ltd. signed a final merger agreement to purchase Good Therapeutics, a US-based biopharmaceutical business, for a cash upfront payment of $250 Million.
D-dimer Testing Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Test Types Covered | Clinical Laboratory Tests, Point-of-Care Tests |
| Products Covered | Analyzers, Reagents and Consumables |
| Methods Covered | Enzyme-linked Immunosorbent Assay (ELISA), Latex-enhanced Immunoturbidimetric Assays, Fluorescence Immunoassays, Others |
| Applications Covered | Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Others |
| End Uses Covered | Hospitals, Academic and Research Institutes, Diagnostic Centers, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Abbott Laboratories, BioMedica Diagnostics, biomérieux SA, Diazyme Laboratories Inc. (General Atomics), F. Hoffmann-La Roche Ltd., HORIBA Ltd., Quidel Corporation, Sekisui Diagnostics LLC (Sekisui Chemical Co. Ltd.), Siemens Healthcare GmbH, Thermo Fisher Scientific Inc., Unbound Medicine Inc., Werfen, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the D-dimer testing market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the global D-dimer testing market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the D-dimer testing industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
Key Questions Answered in This Report
The D-dimer testing market was valued at USD 1.52 Billion in 2025.
The D-dimer testing market is projected to exhibit a CAGR of 3.17% during 2026-2034, reaching a value of USD 2.01 Billion by 2034.
The market is driven by increasing prevalence of venous thromboembolism (VTE), growing awareness of early disease detection, and a rising elderly population. Advances in point-of-care diagnostics, supportive reimbursement policies, and expanding use of D-dimer tests in COVID-19 and cardiovascular assessments are further contributing to the market’s sustained growth.
North America currently dominates the D-dimer testing market in 2025. The dominance is fueled by the region’s high healthcare spending, widespread adoption of advanced diagnostic tools, favorable reimbursement structures, and strong awareness of thrombotic disorders. Robust infrastructure and early integration of testing in emergency and routine care pathways further strengthen regional leadership.
Some of the major players in the D-dimer testing market include Abbott Laboratories, BioMedica Diagnostics, biomérieux SA, Diazyme Laboratories Inc. (General Atomics), F. Hoffmann-La Roche Ltd., HORIBA Ltd., Quidel Corporation, Sekisui Diagnostics LLC (Sekisui Chemical Co. Ltd.), Siemens Healthcare GmbH, Thermo Fisher Scientific Inc., Unbound Medicine Inc., and Werfen, among others.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












