Dysmenorrhea Market Size to Reach USD 20.0 Billion by 2035, Impelled by Advancements in Pain Management & Pharmaceuticals
Dysmenorrhea Market Outlook 2025-2035:
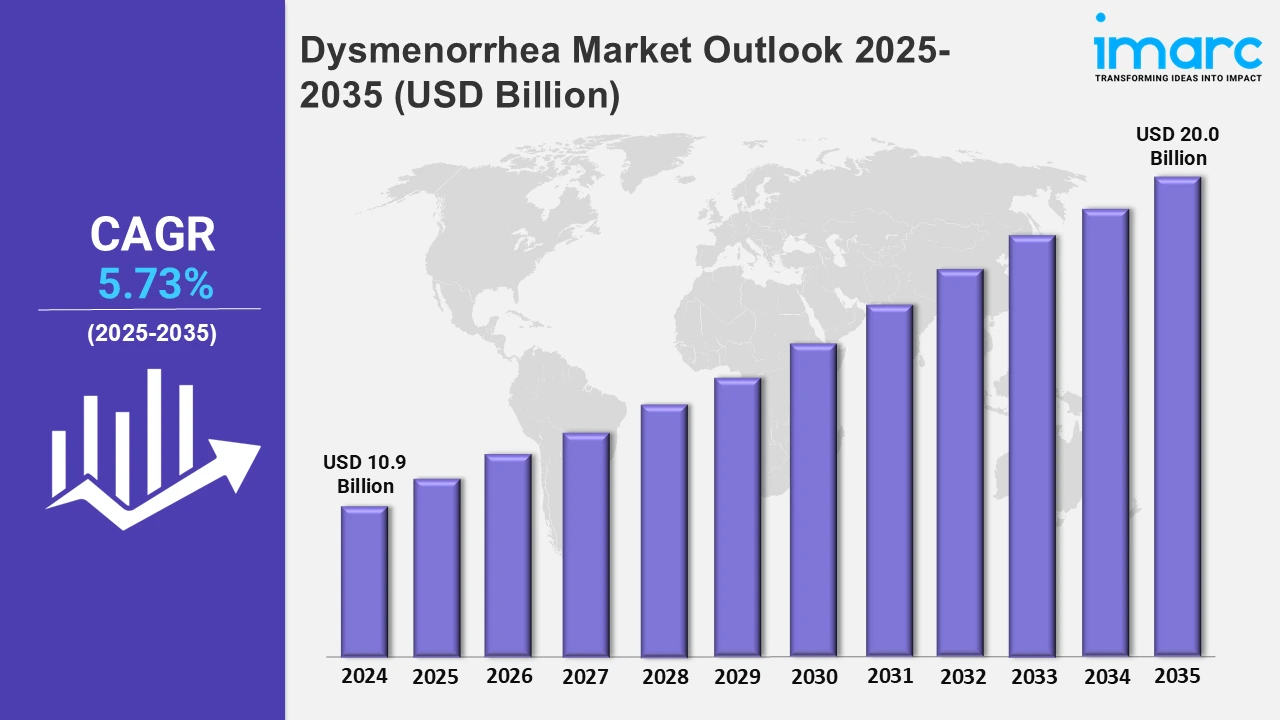
The Dysmenorrhea market reached a value of USD 10.9 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 20.0 Billion by 2035, exhibiting a growth rate (CAGR) of 5.73% during 2025-2035. The dysmenorrhea treatment market is experiencing a dynamic shift, fueled by progress in both diagnostics and therapeutic interventions. The early and accurate diagnosis of dysmenorrhea is increasingly being made possible using modern technologies such as hormonal profiling and imaging through ultrasound, as well as AI menstrual health applications. All these give healthcare providers the ability to distinguish between primary and secondary dysmenorrhea, such that they can design tailored treatment strategies or may provide early intervention for improved patient journeys. It further transforms the therapeutic side of the dysmenorrhea management market with emerging new treatment options. Although conventional NSAIDs and hormones still play their parts, new options with non-hormonal medication have emerged to offer solutions with potentially lesser side effects. In addition, combining a pain reliever with a neuromodulation device or targeted hormonal treatments improves symptom control and makes pain relief and quality of life more bearable. These advancements are transforming dysmenorrhea management for millions worldwide.
To get more information on this market, Request Sample
Increasing Awareness & Diagnosis Rates
Greater increases in awareness and educational programs and initiatives concerning the subject of menstrual health empower many women to regard dysmenorrhea as a condition that can be treated rather than an unavoidable concomitant of their menstrual cycle. Government health agencies and NGOs seek to spread awareness of menstrual health through, among others, school programs, social media campaigns, and community workshops. This has, in turn, resulted in a rise in consultations with doctors, thus stimulating early intervention and better management of menstrual pain. Meanwhile, advances in diagnostics, like high-resolution ultrasound, MRI, and hormonal assessment, have provided opportunities to ascertain with accuracy underlying anomalies such as endometriosis, adenomyosis, and fibroids. The advent of many AI-driven menstrual tracking applications has given increasing power to the early diagnosis of dysmenorrhea via pain pattern monitoring and potential prediction of reproductive health disorders. Given the heightened awareness and precision in diagnostics, the number of women now receiving individualized treatment options has multiplied, therefore improving the quality of life and long-term health outcomes.
Increasing Adoption of Alternative & Non-Invasive Therapies
The ongoing trend among women is the preference for natural, noninvasive treatments for dysmenorrhea with fewer side effects, especially safer options. Herbal supplements, such as ginger, turmeric, and chasteberry, are in vogue because of their reputed anti-inflammatory and pain-relieving properties. Acupuncture and acupressure practices are under consideration for helping with hormonal balance and menstrual pain relief. Changes in dietary habits, which include increased intake of omega-3s and decreased consumption of caffeine, are more beneficial in assisting individuals with inflammation and cramps. Also, wearable devices for pain relief, such as transcutaneous electrical nerve stimulation (TENS) units, have been much sought after in assisting women with back pain through stimulation of nerves and better blood circulation without drugs. Yoga and meditation techniques focusing on reducing stress and relaxing muscles are now merging with a holistic approach toward dysmenorrhea management. With consumer awareness and technological development, the acceptance of alternative and noninvasive therapies is expected to expand further, offering more personalized and sustainable treatment for women.
Marketed Therapies in the Dysmenorrhea Market
Naprelan (Naproxen sodium) - Almatica Pharma
Naprelan (Naproxen Sodium), developed by Almatica Pharma, is a widely used extended-release NSAID for managing dysmenorrhea by reducing inflammation and relieving menstrual pain. Its long-acting formulation provides sustained pain relief, minimizing the need for frequent dosing.
Emerging Therapies in the Dysmenorrhea Market
DARE PDM1 - Dare Bioscience
DARE-PDM1, developed by Dare Bioscience, is an innovative, non-hormonal treatment in development for dysmenorrhea, aiming to provide effective and long-lasting pain relief. Designed as a novel, extended-release formulation, it targets menstrual pain by modulating prostaglandin activity, a key driver of uterine contractions.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| DARE PDM1 | Dare Bioscience | Cyclooxygenase inhibitors | Oral |
Detailed list of emerging therapies for dysmenorrhea provided in the final report…
Leading Companies in the dysmenorrhea Market:
The dysmenorrhea treatment market is undergoing a significant transformation, driven by competition and breakthrough innovations from leading pharmaceutical and biotech companies. Key players such as Almatica Pharma and Dare Bioscience are heavily investing in advanced pain management solutions, hormonal therapies, and non-invasive treatments to improve the management of menstrual pain. A major shift is occurring, moving beyond traditional NSAIDs and oral contraceptives toward disease-modifying treatments that specifically target uterine contractions, inflammation, and hormonal imbalances driving dysmenorrhea. This is evident in the development of novel therapies such as Naprelan (Naproxen Sodium), DARE-PDM1, and next-generation hormonal modulators, which aim to enhance treatment efficacy, reduce side effects, and provide long-lasting relief. Supported by ongoing clinical trials, innovative drug delivery systems, and digital health solutions, the dysmenorrhea treatment landscape is evolving toward personalized and effective therapies, offering hope for better pain control and improved quality of life for millions of affected women.
Key Players in the dysmenorrhea Market:
The key players in the dysmenorrhea market who are in different phases of developing different therapies are Almatica Pharma, Dare Bioscience, and others.

Regional Analysis:
The dysmenorrhea treatment market is heavily concentrated in developed regions such as the United States, Germany, the UK, France, Italy, Spain, and Japan, where advancements in women’s health research and pain management therapies are driving innovation. The United States plays a pivotal role in this market due to its leadership in pharmaceutical R&D, digital health solutions, and non-invasive treatment technologies for menstrual pain. While current treatment approaches focus on NSAIDs, hormonal therapies, and lifestyle modifications, significant progress is being made in understanding the underlying causes of dysmenorrhea, leading to the development of targeted and non-hormonal therapies. The market is also being fueled by increased investments in women's health research, accelerated regulatory approvals for innovative pain management solutions, and strategic collaborations between pharmaceutical companies, research institutions, and healthcare providers. These efforts are expanding treatment options, improving menstrual health management, and ultimately enhancing quality of life for women affected by dysmenorrhea.
Key information covered in the report.
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the dysmenorrhea market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the dysmenorrhea market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current dysmenorrhea-marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












