
Idiopathic Pulmonary Fibrosis Treatment Market Size, Share, Trends and Forecast by Drug Class, End User, and Region, 2025-2033
Idiopathic Pulmonary Fibrosis Treatment Market Size and Share:
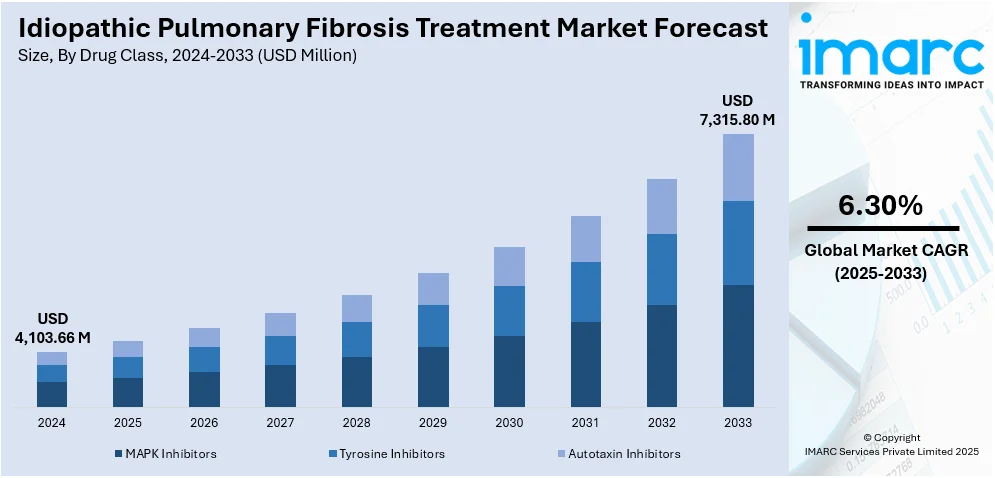
The global idiopathic pulmonary fibrosis treatment market size was valued at USD 4,103.66 Million in 2024. Looking forward, the market is expected to reach USD 7,315.80 Million by 2033, exhibiting a CAGR of 6.30% during 2025-2033. North America currently dominates the market, holding a significant market share of 37.5% in 2024. The market is driven by rising disease awareness, advancements in antifibrotic therapies, increasing research into combination treatments, expanding diagnostic capabilities, and supportive regulatory frameworks. Pharmaceutical innovation and strategic collaborations among key players continue to shape the competitive landscape of the idiopathic pulmonary fibrosis treatment market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 4,103.66 Million |
| Market Forecast in 2033 | USD 7,315.80 Million |
| Market Growth Rate (2025-2033) | 6.30% |
The increasing global prevalence of IPF, particularly among the aging population, is significantly boosting demand for effective therapies, fueling the market growth. Advances in diagnostic imaging and early detection technologies are enabling timely intervention, leading to improved treatment outcomes. The development and approval of novel antifibrotic drugs, alongside ongoing clinical trials for combination therapies, are expanding therapeutic options. Additionally, growing awareness about the disease among patients and healthcare professionals has accelerated early consultations and diagnosis rates. Supportive regulatory policies and rising healthcare spending in developed and emerging markets are also enhancing access to treatments.
To get more information on this market, Request Sample
The idiopathic pulmonary fibrosis treatment market growth in the United States is driven by the rising prevalence of the disease, particularly among the aging population. Early diagnosis through advanced imaging technologies and increased disease awareness among healthcare providers are enabling timely treatment. The availability of approved antifibrotic drugs like nintedanib and pirfenidone, coupled with a robust clinical pipeline exploring next-generation therapies, is enhancing treatment options. Additionally, strong healthcare infrastructure, favorable insurance coverage, and high research and development (R&D) investment by pharmaceutical companies support market expansion. Government support for rare disease research and growing patient advocacy are further contributing to the growth of IPF treatment adoption across the US. For instance, in October 2023, Bristol Myers Squibb revealed that the US Food and Drug Administration (FDA) granted Breakthrough Therapy Designation to BMS-986278, its experimental oral treatment and a novel lysophosphatidic acid receptor 1 (LPA1) antagonist. The designation applies to the treatment of progressive pulmonary fibrosis (PPF), a serious and life-threatening condition for which only one approved therapy currently exists.
Idiopathic Pulmonary Fibrosis Treatment Market Trends:
Rising Prevalence of IPF and Aging Population
One of the primary drivers of the IPF treatment market is the increasing prevalence of the disease, particularly among the elderly. Idiopathic pulmonary fibrosis is more common in individuals over 60, and as global life expectancy rises, the patient population continues to grow. Aging also increases vulnerability to lung damage and fibrotic changes, heightening demand for diagnostic evaluations and therapeutic intervention. Additionally, improved awareness and screening practices have led to more cases being detected at earlier stages. As highlighted by the American Lung Association, 15.4% of adults in rural communities smoke, compared to 10.1% in urban areas, pointing to a higher risk exposure and greater need for effective treatment solutions in these regions. As the number of at-risk individuals expands, the need for long-term care and disease management solutions grows in parallel, thereby fueling demand for both existing and emerging IPF therapies across global healthcare systems.
Advancements in Antifibrotic Therapies and Drug Development
The introduction of antifibrotic drugs such as pirfenidone and nintedanib has significantly transformed the treatment landscape for IPF by slowing disease progression and improving quality of life. According to the idiopathic pulmonary fibrosis treatment market trends, their success has spurred pharmaceutical investment in developing next-generation therapies, including inhaled formulations, combination regimens, and novel drug classes such as LPA1 antagonists. Ongoing clinical trials, including those targeting molecular pathways like TGF-β and integrin signaling, are expected to broaden the therapeutic toolkit. Additionally, regulatory agencies worldwide are supporting fast-track designations and orphan drug approvals for innovative treatments, accelerating their time to market. This momentum in drug discovery and approval processes is a critical growth engine for the IPF treatment market, expanding choices for clinicians and patients alike. For instance, in May 2025, Cumberland Pharmaceuticals Inc., a specialty drug manufacturer concentrating on treatments for rare disorders, announced a strategic alliance with Qureight, an advanced imaging laboratory specializing in deep-learning-based image analytics. This collaboration is designed to improve the quality and interpretation of data emerging from Cumberland’s FIGHTING FIBROSIS™ Phase II clinical study. The trial focuses on assessing the efficacy of ifetroban, Cumberland’s investigational therapy, in individuals diagnosed with idiopathic pulmonary fibrosis (IPF), the most prevalent type of progressive fibrosing interstitial lung disease.
Improved Diagnostic Capabilities and Early Detection
Early diagnosis of IPF significantly improves treatment outcomes, and advancements in diagnostic tools are creating a positive idiopathic pulmonary fibrosis treatment market outlook. High-resolution computed tomography (HRCT), pulmonary function tests, and multidisciplinary assessment protocols now enable more accurate and earlier identification of IPF cases. Enhanced imaging technologies and biomarker research are also helping distinguish IPF from other interstitial lung diseases, reducing diagnostic delays. Moreover, rising awareness among general practitioners and pulmonologists has improved referral rates to specialists, enabling timely intervention. Early-stage diagnosis allows for earlier initiation of antifibrotic therapies, which can more effectively slow disease progression. As diagnostic accuracy improves, patient volumes for treatment increase, thus contributing significantly to the expansion of the IPF treatment market. For instance, in November 2024, Endeavor BioMedicines, a biotechnology firm in the clinical development stage focused on innovative therapies for serious illnesses, announced the dosing of the first participant in its Phase 2b WHISTLE-PF study. This trial is designed to assess the safety and effectiveness of ENV-101 (taladegib), the company’s primary experimental drug, in patients diagnosed with idiopathic pulmonary fibrosis (IPF). ENV-101 is a first-in-class inhibitor targeting the Hedgehog signaling pathway and has shown encouraging results in a prior Phase 2a trial, positioning it as a potential disease-modifying therapy for IPF, one of the most challenging progressive lung diseases.
Idiopathic Pulmonary Fibrosis Treatment Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global idiopathic pulmonary fibrosis treatment market, along with forecasts at the global, regional, and country levels from 2025-2033. The market has been categorized based on drug class and end user.
Analysis by Drug Class:
- MAPK Inhibitors
- Tyrosine Inhibitors
- Autotaxin Inhibitors
Tyrosine inhibitors hold the largest share in the idiopathic pulmonary fibrosis (IPF) treatment market primarily due to the widespread use and proven efficacy of drugs like nintedanib. Nintedanib, a multi-targeted TKI, slows disease progression by inhibiting pathways involved in fibrogenesis, including VEGF, PDGF, and FGF receptors. Its approval in multiple regions, including the U.S., Europe, and Asia, and inclusion in clinical guidelines have driven strong physician adoption. Additionally, TKIs are supported by robust clinical evidence, favorable safety profiles, and long-term outcome data. Their established role in IPF management and limited competition from other drug classes further solidify their dominant market position.
Analysis by End User:
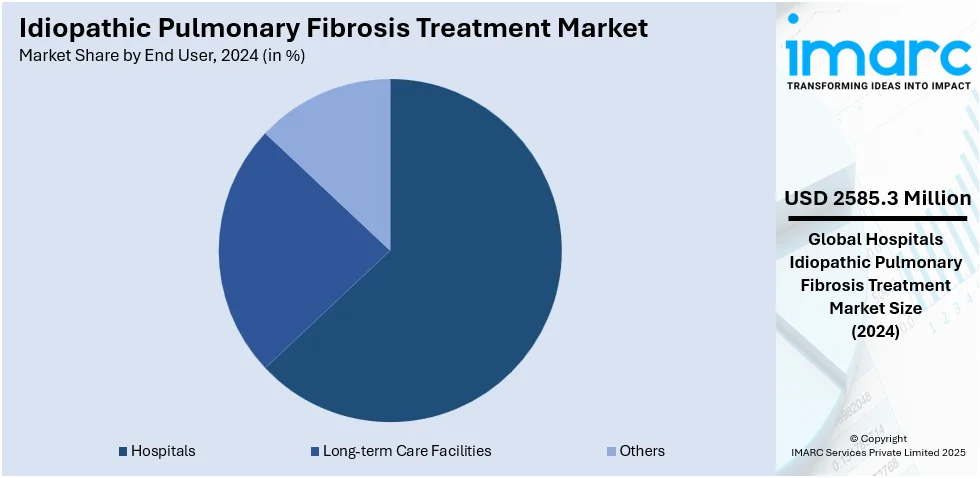
- Hospitals
- Long-term Care Facilities
- Others
Hospitals lead the market with 63.0% of market share in 2024 due to their central role in diagnosis, management, and advanced care delivery. IPF is a complex, progressive disease that requires multidisciplinary expertise, including pulmonologists, radiologists, and respiratory therapists, resources typically concentrated in hospital settings. Hospitals are equipped with high-resolution CT scanners, pulmonary function testing, and specialized labs essential for accurate diagnosis and disease monitoring. They also administer antifibrotic therapies, manage comorbidities, and handle acute exacerbations. According to the idiopathic pulmonary fibrosis treatment market forecast, hospitals often serve as trial sites for emerging treatments, further reinforcing their dominance in IPF care delivery and overall market share.
Regional Analysis:
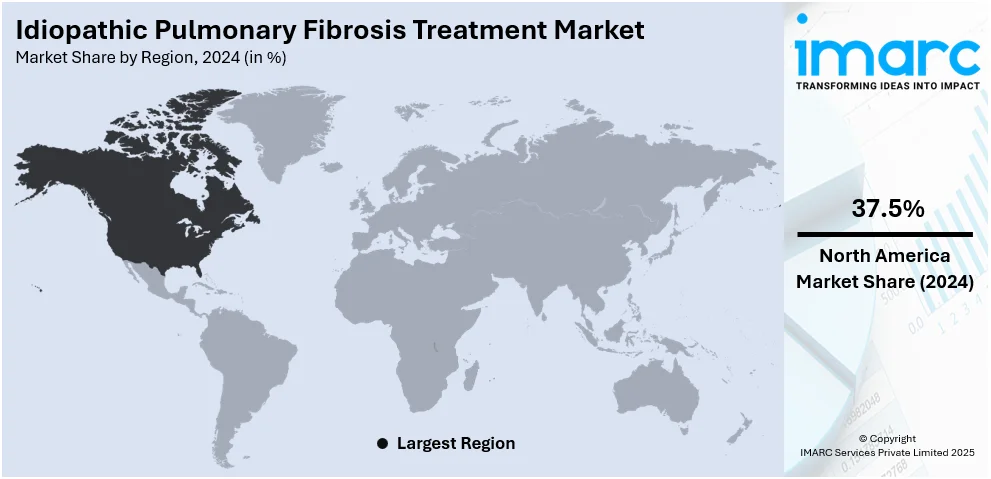
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
In 2024, North America accounted for the largest market share of 37.5%. The idiopathic pulmonary fibrosis treatment market demand in North America is driven by the region’s aging population, as IPF primarily affects individuals over 60. High disease awareness among healthcare providers and patients also leads to earlier diagnosis and treatment. The presence of advanced healthcare infrastructure supports widespread use of diagnostic tools like high-resolution CT scans and pulmonary function tests. Moreover, the availability and reimbursement of approved antifibrotic therapies, such as nintedanib and pirfenidone, promote consistent treatment adoption. Active research and clinical trials in the U.S. and Canada further expand therapeutic options. Strong regulatory support, combined with rising investment from pharmaceutical companies, continues to strengthen North America’s position in the global IPF treatment market.
Key Regional Takeaways:
United States Idiopathic Pulmonary Fibrosis Treatment Market Analysis
In 2024, the United States accounted for 88.2% of the idiopathic pulmonary fibrosis treatment market in North America. The United States idiopathic pulmonary fibrosis (IPF) treatment market is experiencing robust growth due to increased awareness about pulmonary disorders and advancements in early diagnostic techniques. The widespread adoption of telehealth services is enabling quicker patient access to respiratory specialists, promoting timely disease management. The growing elderly population, which is more susceptible to fibrotic lung conditions, is significantly contributing to the market expansion. Moreover, there is an increasing emphasis on personalized medicine and biomarker-based therapies, which is fostering innovation in targeted treatment approaches. According to a recent report, an estimated 32 million individuals in the United States are living with chronic obstructive pulmonary disease (COPD), a widespread and persistent respiratory condition, highlighting the substantial burden of respiratory illnesses and reinforcing the need for enhanced fibrotic lung disease management. The presence of advanced research infrastructure has led to continuous clinical trials and the development of novel therapeutics. The increasing integration of AI-powered radiology for detecting interstitial lung abnormalities is further streamlining patient identification and follow-up care. The US IPF treatment market is growing due to increased treatment adherence rates, patient support programs, and strategic collaborations between research institutions and public health entities, fostering comprehensive care models.
Europe Idiopathic Pulmonary Fibrosis Treatment Market Analysis
The idiopathic pulmonary fibrosis treatment market in Europe is witnessing steady growth, driven by the expansion of universal healthcare services that improve access to pulmonary care. Enhanced public health initiatives and investments in respiratory disease surveillance are contributing to the early identification and management of IPF cases. A growing focus on environmental health and occupational safety standards is increasing public awareness about respiratory risks, thereby facilitating earlier diagnosis. Notably, the UK government reported a 7% rise in emergency admissions for respiratory diseases in England between the financial years ending 2023 and 2024. This upward trend emphasizes the growing urgency to implement proactive treatment strategies and strengthen healthcare responses to manage pulmonary conditions more effectively. The rise in outpatient pulmonary rehabilitation centers is supporting disease management through non-pharmacological interventions alongside drug therapies. IPF's inclusion in rare disease frameworks boosts policy support, research funding, clinical protocol development, multidisciplinary care, and real-world data integration, improving disease progression and therapeutic effectiveness.
Asia Pacific Idiopathic Pulmonary Fibrosis Treatment Market Analysis
In the Asia Pacific region, the idiopathic pulmonary fibrosis treatment market is expanding due to increasing investments in healthcare infrastructure and a rising focus on chronic respiratory disease management. The growing use of mobile health technologies and wearable devices is aiding in patient monitoring and timely intervention. Urbanization-related environmental changes, including deteriorating air quality, contribute to heightened surveillance and diagnosis of fibrotic lung conditions. According to reports, the entire population of India approximately 1.4 Billion people lives in areas where PM2.5 levels exceed WHO guidelines, exposing them annually to pollution levels that can adversely impact lung health. This alarming statistic underscores the urgent need for improved respiratory diagnostics and interventions in the region. Medical associations' educational outreach, digital imaging tools, integrative care approaches, and government-led health campaigns are enhancing awareness about lung health issues, leading to quicker referrals to specialists and increased demand for IPF treatment options.
Latin America Idiopathic Pulmonary Fibrosis Treatment Market Analysis
The Latin American idiopathic pulmonary fibrosis treatment market is gaining momentum due to increased research, public-private partnerships, and rural health initiatives introducing mobile diagnostic units. The growing adoption of electronic health records is streamlining case tracking and follow-ups, supporting consistent patient management. According to the ministry reports, a rise in the proportion of adult smokers from 9.3% to 11.6% is intensifying the focus on respiratory health and encouraging earlier diagnosis and intervention strategies for conditions like IPF. Health literacy campaigns tailored to local populations are encouraging earlier health-seeking behavior, which benefits the identification and treatment of chronic respiratory conditions like IPF. Rising training initiatives for healthcare professionals in interstitial lung diseases are strengthening the capacity of the regional health systems to manage complex cases, thereby supporting steady market growth.
Middle East and Africa Idiopathic Pulmonary Fibrosis Treatment Market Analysis
The idiopathic pulmonary fibrosis treatment market in the Middle East and Africa is gaining traction with the emergence of regional pulmonary health programs and increased focus on diagnostic capacity-building. Expansion of urban healthcare networks is providing wider access to specialized respiratory services, particularly in metropolitan areas. The incidence of IPF increases with age and is generally higher in men than women. A study from Saudi Arabia found that 23% out of 330 ILD patients have IPF. This alarming data underscores the urgent need for enhanced respiratory care, indirectly bolstering the IPF treatment market. Mobile health screening, medical education programs, and philanthropic initiatives are enhancing the identification of fibrotic lung conditions in semi-urban and rural populations, promoting market development.
Competitive Landscape:
The idiopathic pulmonary fibrosis (IPF) treatment market features a moderately competitive landscape dominated by key players such as Boehringer Ingelheim and Roche, which lead with approved antifibrotic drugs, nintedanib (Ofev) and pirfenidone (Esbriet), respectively. These therapies have set the standard for disease management, maintaining a strong foothold in global markets. However, increasing R&D activity is intensifying competition, with companies like Bristol Myers Squibb, Pliant Therapeutics, and FibroGen developing next-generation treatments targeting novel pathways such as LPA1 antagonists and integrin inhibitors. Strategic collaborations, licensing deals, and regulatory designations are common across the landscape. Despite clinical setbacks, innovation remains robust, driven by the urgent need for disease-modifying therapies. The pipeline’s expansion is reshaping the future dynamics of the IPF treatment market.
The report provides a comprehensive analysis of the competitive landscape in the idiopathic pulmonary fibrosis (IPF) treatment market with detailed profiles of all major companies, including:
- Boehringer Ingelheim Pharmaceuticals, Inc.
- Cipla Inc.
- F. Hoffmann-La Roche Ltd
- GNI Group Ltd
- Lupin Ltd
Latest News and Developments:
- June 2025: Insilico Medicine published Phase IIa results of Rentosertib, an AI-designed TNIK inhibitor, showing a +98.4 mL improvement in lung function in IPF patients. The drug demonstrated safety, anti-fibrotic effects, and biomarker validation, marking a major milestone in AI-driven drug development and offering hope for disease-modifying IPF therapies.
- May 2025: Endeavor BioMedicines presented post hoc Phase 2a results of ENV-101 at ATS 2025, showing significant lung volume gains and pulmonary vessel volume reduction in IPF patients. Deep learning CT analysis supported reduced fibrosis trends, reinforcing ENV-101’s disease-modifying potential and paving the way for continued evaluation in the WHISTLE-PF trial.
- May 2025: Rein Therapeutics initiated its Phase II RENEW trial of LTI-03 for IPF, enrolling up to 120 patients globally. The study evaluated inhaled LTI-03’s safety, efficacy, and biomarker impact. Building on Phase Ib results, the trial aimed to assess alveolar cell survival and antifibrotic signaling inhibition using Qureight-supported imaging.
- May 2025: The FIBRONEER-IPF and FIBRONEER-ILD trials showed that nerandomilast significantly slowed lung function decline in IPF patients over a year. Involving over 2,200 participants, the drug was well tolerated and effective alone or with existing therapies, marking the first promising new treatment in over a decade.
- April 2025: Tulane researchers found that the cancer drug ipilimumab improved lung repair in mice with idiopathic pulmonary fibrosis by blocking CTLA-4, enhancing the immune clearance of scar-causing cells. This discovery highlighted a potential new treatment approach, though further studies were needed to confirm safety and efficacy in humans.
Idiopathic Pulmonary Fibrosis Treatment Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Drug Classes Covered | MAPK Inhibitors, Tyrosine Inhibitors, Autotaxin Inhibitors |
| End Users Covered | Hospitals, Long-term Care Facilities, Others |
| Region Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Boehringer Ingelheim Pharmaceuticals, Inc., Cipla Inc., F. Hoffmann-La Roche Ltd, GNI Group Ltd, Lupin Ltd, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Idiopathic pulmonary fibrosis treatment market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the global Idiopathic pulmonary fibrosis treatment market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Idiopathic pulmonary fibrosis treatment industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
The idiopathic pulmonary fibrosis treatment market was valued at USD 4,103.66 Million in 2024.
The idiopathic pulmonary fibrosis treatment market is projected to exhibit a CAGR of 6.30% during 2025-2033, reaching a value of USD 7,315.80 Million by 2033.
The idiopathic pulmonary fibrosis treatment market is driven by a growing elderly population, rising disease awareness, and advancements in antifibrotic therapies. Increased diagnostic capabilities, ongoing clinical research, and strong regulatory support further contribute to market expansion, addressing the urgent need for effective treatments in managing this progressive lung disease.
North America currently dominates the idiopathic pulmonary fibrosis treatment market due to an aging population, advanced diagnostic infrastructure, early disease detection, availability of approved therapies, active clinical trials, and strong healthcare reimbursement policies supporting access to effective antifibrotic treatments.
Some of the major players in the idiopathic pulmonary fibrosis treatment market includes Boehringer Ingelheim Pharmaceuticals, Inc., Cipla Inc., F. Hoffmann-La Roche Ltd, GNI Group Ltd, Lupin Ltd, etc.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












