
India Human Papillomavirus Vaccine Market Size, Share, Trends and Forecast by Valence, Disease Indication, Distribution Channel, and Region, 2025-2033
India Human Papillomavirus Vaccine Market Overview:
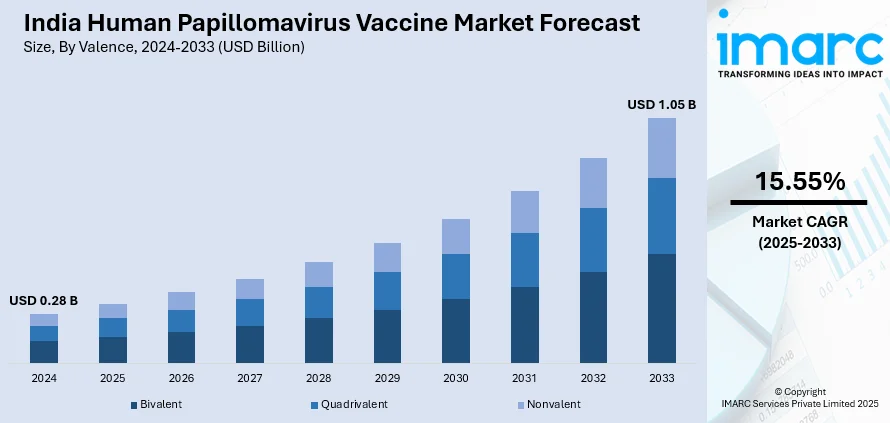
The India human papillomavirus vaccine market size reached USD 0.28 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 1.05 Billion by 2033, exhibiting a growth rate (CAGR) of 15.55% during 2025-2033. The rising awareness regarding cervical cancer, government immunization programs, and increased affordability, owing to domestic vaccine production, are some of the key factors strengthening the market growth. Besides this, growing healthcare infrastructure, support from organizations, such as the WHO, and the push for adolescent vaccination are also bolstering the market growth.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 0.28 Billion |
| Market Forecast in 2033 | USD 1.05 Billion |
| Market Growth Rate 2025-2033 | 15.55% |
India Human Papillomavirus Vaccine Market Trends:
Inclusion of HPV Vaccination in National Immunization Programs
The Indian government’s decision to integrate the HPV vaccine into its Universal Immunization Program (UIP) marks a significant step in strengthening public health initiatives. As announced in the 2024 Union Budget by Finance Minister Nirmala Sitharaman, the program aims to vaccinate girls aged 9 to 14 against cervical cancer, targeting nearly 80 million beneficiaries over three years. Vaccinations will be administered through schools and primary health centers to ensure widespread coverage. Cervical cancer is the second-most prevalent cancer among Indian women, with approximately 125,000 new cases and 75,000 deaths reported annually. By incorporating the quadrivalent HPV vaccine, which provides protection against HPV types 6, 11, 16, and 18, the government seeks to significantly reduce these alarming statistics. This initiative aligns with global efforts to integrate HPV vaccination into national immunization programs, reinforcing India's commitment to preventive healthcare. The proactive approach underscores the importance of early vaccination in combating cervical cancer and improving long-term public health outcomes.
To get more information of this market, Request Sample
Rise of Domestic Vaccine Production
The launch of India’s first indigenous quadrivalent HPV vaccine, CERVAVAC, by the Serum Institute of India (SII) in January 2023 has reshaped the country's HPV vaccine market. This achievement made possible through collaborations between SII, the Department of Biotechnology (DBT), the Biotechnology Industry Research Assistance Council (BIRAC), and the Bill and Melinda Gates Foundation, reinforces India's self-sufficiency in vaccine production. CERVAVAC is expected to make HPV vaccination significantly more affordable and accessible. Previously, vaccines like Gardasil cost between INR 2,000 and INR 4,000 per dose, whereas government negotiations have set CERVAVAC’s price at INR 200 to INR 250 per dose—nearly one-tenth of the previous retail cost. This dramatic price reduction is likely to boost vaccine adoption, particularly in rural and low-income regions. Additionally, domestic production reduces dependence on international suppliers, ensuring a stable supply chain and reinforcing India’s ‘Make in India’ initiative. The successful public-private collaboration behind CERVAVAC highlights the potential of indigenous innovation in tackling critical public health challenges.
India Human Papillomavirus Vaccine Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the region/country level for 2025-2033. Our report has categorized the market based on valence, disease indication, and distribution channel.
Valence Insights:
- Bivalent
- Quadrivalent
- Nonvalent
The report has provided a detailed breakup and analysis of the market based on the valence. This includes bivalent, quadrivalent, and nonvalent.
Disease Indication Insights:
- Cervical Cancer
- Anal Cancer
- Vaginal Cancer
- Penile Cancer
- Vulvar Cancer
A detailed breakup and analysis of the market based on the disease indication have also been provided in the report. This includes cervical cancer, anal cancer, vaginal cancer, penile cancer, and vulvar cancer.
Distribution Channel Insights:
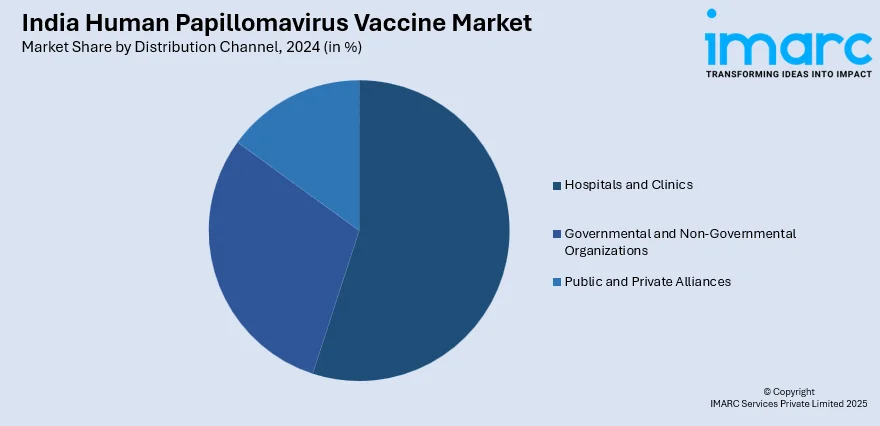
- Hospitals and Clinics
- Governmental and Non-Governmental Organizations
- Public and Private Alliances
The report has provided a detailed breakup and analysis of the market based on the distribution channel. This includes hospitals and clinics, governmental and non-governmental organizations, and public and private alliances.
Regional Insights:
- North India
- South India
- East India
- West India
The report has also provided a comprehensive analysis of all the major regional markets, which include North India, South India, East India, and West India.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
India Human Papillomavirus Vaccine Market News:
- April 2025: Tens of thousands of medical professionals across the country began to receive specific training to promote the HPV vaccine and address cervical cancer. This was supported by organizations such as the Federation of Obstetric and Gynecological Societies of India (FOGSI) and partly funded by Cancer Research UK.
- March 2025: The Pune Municipal Corporation (PMC) announced that it would initiate a Human Papillomavirus (HPV) vaccination program for girls in Classes 8 and 9 across municipal schools from June 2025. PMC plans to collaborate with NGOs and utilize Corporate Social Responsibility (CSR) funds to subsidize the vaccine's cost, ensuring broader access and protection for young girls.
India Human Papillomavirus Vaccine Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Valences Covered | Bivalent, Quadrivalent, Nonvalent |
| Disease Indications Covered | Cervical Cancer, Anal Cancer, Vaginal Cancer, Penile Cancer, Vulvar Cancer |
| Distribution Channels Covered | Hospitals and Clinics, Governmental and Non-Governmental Organizations, Public and Private Alliances |
| Regions Covered | North India, South India, East India, West India |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the India human papillomavirus vaccine market performed so far and how will it perform in the coming years?
- What is the breakup of the India human papillomavirus vaccine market on the basis of valence?
- What is the breakup of the India human papillomavirus vaccine market on the basis of disease indication?
- What is the breakup of the India human papillomavirus vaccine market on the basis of distribution channel?
- What are the various stages in the value chain of the India human papillomavirus vaccine market?
- What are the key driving factors and challenges in the India human papillomavirus vaccine market?
- What is the structure of the India human papillomavirus vaccine market and who are the key players?
- What is the degree of competition in the India human papillomavirus vaccine market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the India human papillomavirus vaccine market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the India human papillomavirus vaccine market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the India human papillomavirus vaccine industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












