Interstitial Cystitis Market Size to Reach USD 3.5 Billion by 2035, Impelled by Advancements in Intravesical and Regenerative Treatments
Interstitial Cystitis Market Outlook 2025-2035:
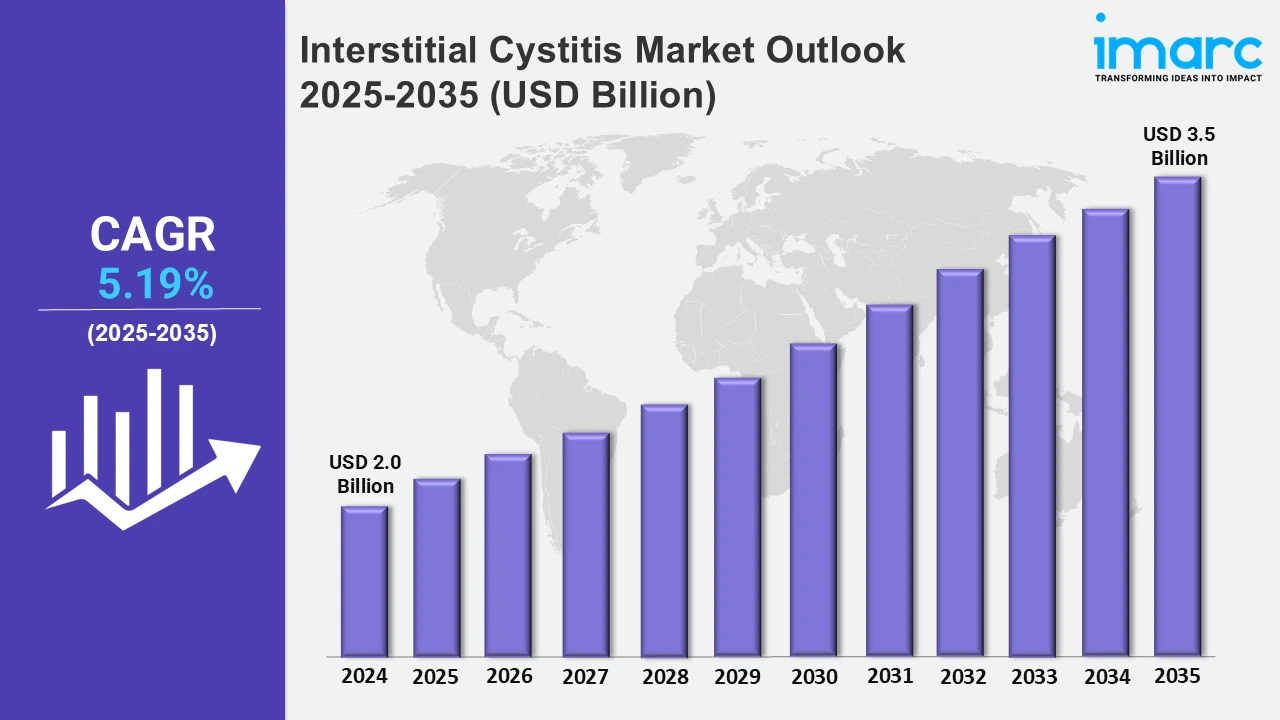
The 7 major interstitial cystitis market reached a value of USD 2.0 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 3.5 Billion by 2035, exhibiting a growth rate (CAGR) of 5.19% during 2025-2035. The Interstitial Cystitis (IC) market is propelled by the growing preference for non-invasive and minimally invasive treatment options, including intravesical therapies, neuromodulation techniques, and bladder instillations, which effectively alleviate IC symptoms while reducing discomfort and recovery time. These innovative approaches are particularly effective in addressing chronic bladder inflammation, relieving pain, and improving bladder function, leading to better patient outcomes and increased satisfaction. By minimizing the need for prolonged oral medication and invasive procedures, these treatments offer patients efficient and convenient solutions with fewer side effects.
To get more information on this market, Request Sample
Advancements in Early Detection and Diagnostic Technologies: Driving the Interstitial Cystitis Market
Modern diagnostic and treatment technologies have revolutionized the interstitial cystitis (IC) market, which has improved patient management and outcomes significantly. Cystoscopy with high definition imaging and OCT allows detailed views of the lining of the bladder and allows for appropriate and individualized planning of treatment. This is combined with biomarkers and urine analysis to determine inflammation markers and pathogen presence in order to achieve an accurate diagnosis of IC. Molecular diagnostics, such as PCR and next-generation sequencing (NGS), have also become increasingly prominent in identifying microbial imbalances and genetic markers related to chronic bladder inflammation, thus forming the foundation for developing personalized therapeutic strategies. Further, AI-driven diagnostic systems enhance precision through the automation of the process of detecting bladder anomalies, measuring symptom severity, and monitoring treatment progress, thereby minimizing the use of assessments involving personal opinions. Non-invasive treatments include techniques such as neuromodulation, advanced intravesical treatments, and hydrodistension via advanced catheters. New wearable technologies are giving rise to the development of products such as bladder activity trackers that can be used for real-time monitoring of bladder function and symptom patterns, allowing for continuous outpatient care and timely interventions. Such advancements are particularly beneficial in areas where there is a scarcity of urologists, ensuring early IC symptom detection and effective management. The rise of telemedicine also plays a vital role as it can expand care access to remote consultations, symptom monitoring, and tailor-made treatment recommendations. Thus, all these technological advancements aim at improved patient satisfaction, fewer complications, and better long-term outcomes for patients living with IC.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The Interstitial Cystitis (IC) market is growing significantly with the advent of new therapies, coupled with advanced pharmacological treatments. New oral and intravesical agents are under development to treat chronic bladder inflammation and its symptoms, thus becoming crucial in the prevention and management of IC. These innovative treatments show improved efficacy, fewer side effects, a more targeted mechanism of action, and improved patient satisfaction and outcomes. There is also rapid research being done on biological therapies, especially for the moderate to severe cases of IC with chronic inflammatory components. Monoclonal antibodies targeting pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-17 (IL-17), and tumor necrosis factor-alpha (TNF-α) are under study to reduce inflammation and relieve bladder pain and discomfort. Advances in drug delivery systems include liposomal formulations, hydrogels, and nanotechnology-based carriers, where the drug can be delivered locally into the bladder with higher concentrations at the site of action, but with decreased systemic exposure and potential side effects. Complementary therapies in development involve restoring health and immune balance in the bladder through probiotics and immunomodulators to help modulate natural defenses within the bladder, which decreases inflammation. The combination of anti-inflammatory agents along with bladder protective compounds appears promising for the myriad pathophysiologies that comprise IC. Non-invasive pharmaceutical interventions, such as biofilm disruptive agents and novel topical formulations for instillation into the bladder, are also gaining popularity because they make it easier to administer and are patient-centric. All these advancements put together constitute a paradigm shift in the management of IC, promoting better therapeutic outcomes and quality of life for patients.
Marketed Therapies in Interstitial Cystitis Market
Elmiron (Pentosan polysulfate sodium): Bene-Arzneimittel/ReqMed
Elmiron (pentosan polysulfate sodium) is the only FDA-approved oral medication specifically for Interstitial Cystitis (IC). It works by forming a protective layer on the bladder wall, reducing irritation and alleviating pain and discomfort. However, long-term use has been linked to potential retinal toxicity, requiring regular eye examinations.
Emerging Therapies in Interstitial Cystitis Market
Botox: AbbVie
Botox (OnabotulinumtoxinA) is under investigation for the treatment for Interstitial Cystitis (IC) and bladder pain syndrome in cases unresponsive to other therapies. It works by blocking nerve signals that trigger bladder muscle contractions, reducing pain and urinary urgency. Botox is typically administered via bladder injections and offers relief for several months.
SI722: Seikagaku Corporation
SI-722 is an investigational drug developed by Seikagaku Corporation for the treatment of Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS). Administered via intravesical instillation, SI-722 is designed to alleviate symptoms such as frequent urination and bladder pain by releasing a steroid with anti-inflammatory properties directly into the bladder. A Phase I/II randomized, double-blind, placebo-controlled clinical trial has been completed to evaluate its pharmacokinetics, safety, tolerability, and preliminary efficacy in patients with IC/BPS.
URG101: Urigen Pharmaceuticals
URG101 is a combination intravesical therapy containing lidocaine and heparin developed for the treatment of Interstitial Cystitis (IC). It works by providing pain relief through the anesthetic properties of lidocaine while heparin helps protect and repair the bladder lining. Despite showing initial promise, its clinical development faced challenges, limiting its widespread adoption.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Botox | AbbVie | Membrane transport protein modulators; Neuromuscular blocking agents | Bladder instillation |
| SI722 | Seikagaku Corporation | Chondroitin sulfate-steroid conjugate | Intravesically instillation |
| URG101 | Urigen Pharmaceuticals | Factor Xa inhibitors; Sodium channel antagonists; Thrombin inhibitors | Bladder instillation |
Detailed list of emerging therapies in Interstitial Cystitis is provided in the final report.
Leading Companies in the Interstitial Cystitis Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Interstitial Cystitis market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Interstitial Cystitis. Some of the major players include Bene-Arzneimittel/ReqMed, AbbVie, Seikagaku Corporation, Urigen Pharmaceuticals, and others. These companies are driving innovation in the Interstitial Cystitis market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Interstitial Cystitis.
In December 2022, Imbrium Therapeutics, a subsidiary of Purdue Pharma announced the completion of the first patient visit in its Phase 1 clinical study evaluating IMB-150 (also known as LYT-503) as a potential non-opioid treatment for female patients with Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS). Developed by PureTech using its AlivioTM Technology Platform, IMB-150 is a novel formulation of lidocaine. The clinical development program for this treatment is named GainMe-IC, and IMB-150 was licensed from PureTech.
Key Players in Interstitial Cystitis Market:
The key players in the Interstitial Cystitis market who are in different phases of developing different therapies are Bene-Arzneimittel/ReqMed, AbbVie, Seikagaku Corporation, Urigen Pharmaceuticals, and Others.

Regional Analysis:
Major markets for Interstitial Cystitis include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. As per estimates by IMARC, the United States has the highest number of patients suffering from Interstitial Cystitis, which also constitutes the largest market for its treatment. Advanced novel treatment approaches such as biologic agents, antimicrobial peptides, and biofilm disruptors have been developed to act more efficiently than previous modalities at reducing IC bladder inflammation. These therapies also promote protection over the damaged surface by rejuvenation of a functionally effective urinary bladder epithelial lining along with modulation of host immune mechanisms toward relief and the prevention of an episode or relapse of symptomatology. New innovations, including liposomal formulations and nanoparticle-based carriers, are also being developed to enhance drug targeting and delivery, reducing systemic exposure in an aim to achieve improved therapeutic outcomes.
The most recent progress in diagnostic equipment and techniques includes high-resolution cystoscopy, urine biomarkers, and molecular diagnostics such as PCR and NGS, making it possible to diagnose IC earlier and with more accuracy, including its contributors. Such innovation enables a more targeted, customized treatment approach that limits adverse effects and increases positive patient outcomes. Other drivers for the IC market are the release of new guidelines on treatments, investment in R&D, and increasing collaborations among pharmaceutical companies, diagnostic technology companies, and research institutions. AI-powered diagnostic tools and telemedicine platforms are also expanding access to sophisticated care in geographically remote or underserved regions, so patients can receive care in a timely and effective manner. Advanced therapies and diagnostic solutions are the major drivers of the IC market growth in North America and Europe, which are ahead of other regions.
Recent Developments in Interstitial Cystitis Market:
- In August 2021, Imbrium Therapeutics, a subsidiary of Purdue Pharma, announced that it has exercised its option to acquire a worldwide exclusive license from PureTech Health to develop, manufacture, and commercialize IMB-150 (previously known as ALV-107), a non-opioid treatment being developed for Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS). IMB-150 was developed using PureTech’s AlivioTM technology platform.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Interstitial Cystitis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Interstitial Cystitis market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Interstitial Cystitis marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












