
Japan Medical Wearables Market Size, Share, Trends and Forecast by Product, Device Type, End User, and Region, 2026-2034
Japan Medical Wearables Market Overview:
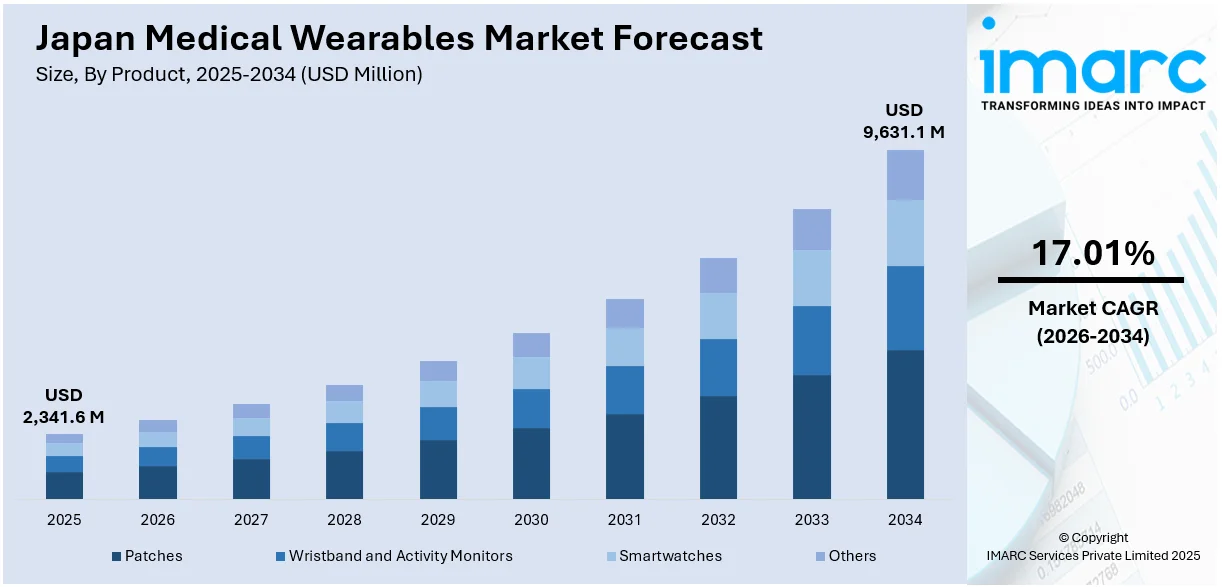
The Japan medical wearables market size reached USD 2,341.6 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 9,631.1 Million by 2034, exhibiting a growth rate (CAGR) of 17.01% during 2026-2034. Advancements in wearable technology, including improvements in materials, sensors, and battery life, are enhancing the comfort, accuracy, and functionality of medical devices. Additionally, regulatory approvals ensure that new devices meet safety standards, boosting trust and accelerating adoption across healthcare settings, thereby contributing to the expansion of the Japan medical wearables market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025
|
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 2,341.6 Million |
| Market Forecast in 2034 | USD 9,631.1 Million |
| Market Growth Rate 2026-2034 | 17.01% |
Japan Medical Wearables Market Trends:
Advancements in Wearable Technology
The ongoing advancements in materials, sensors, and electronics are greatly improving the performance, comfort, and functionality of medical devices. Improvements in flexible and stretchable electronics enable wearables to conform more closely to the body, thereby enhancing comfort and precision for prolonged health tracking. Innovative materials, such as flexible circuits and advanced battery technologies, enable devices to become lighter, more resilient, and capable of prolonged usage without compromising performance. These innovations in wearables enhance patient adherence and broaden the scope of medical uses, including vital sign tracking, chronic condition management, and the oversight of intricate medical procedures. Furthermore, the incorporation of multi-sensing features, like simultaneous monitoring of heart rate, oxygen levels, and neural activity, enhances the overall value of wearables in providing an extensive health profile. With the increasing accuracy, comfort, and features of wearable devices, both healthcare providers and individuals are adopting them more frequently. These technological advancements are creating a basis for more tailored, effective, and user-friendly health management tools, which further strengthen the increasing demand for medical wearables. In 2024, Murata Manufacturing introduced a Stretchable Printed Circuit (SPC) technology that enhances wearable medical devices by offering flexibility, stretchability, and durability. This innovation improved patient comfort and data accuracy for continuous monitoring in devices like EEG, EMG, and ECG. Murata’s SPC supported custom designs and multi-sensing functions for advanced medical applications.
To get more information on this market Request Sample
Regulatory Approvals
Regulatory clearances for sophisticated medical devices are positively influencing the Japan medical wearables market growth. The endorsement of groundbreaking wearable technologies by regulatory agencies, including the Pharmaceuticals and Medical Devices Agency (PMDA), guarantees that new devices adhere to stringent safety and effectiveness criteria, thereby enhancing trust among individuals and healthcare professionals. With regulatory recognition for medical wearables, especially in specific fields, such as cardiac care, the use of these devices is increasing in clinical settings. The endorsement of cutting-edge devices, including those utilizing artificial intelligence (AI) for instant diagnostics and continuous monitoring, creates new opportunities for the market growth. In Japan, where the healthcare system focuses on accuracy and technological integration, these approvals are crucial for the success of wearable health technologies. By simplifying the route to market for advanced technologies, regulatory agencies promote innovation and facilitate industry expansion. With rising regulatory approvals for various wearable devices in medical applications, they are emerging as reliable resources for both doctors and patients, leading to higher adoption rates and greater acceptance in the healthcare industry. The growing trend of regulatory backing for wearable technologies is a key factor driving the market forward, enhancing the accessibility and efficacy of these devices in addressing various health issues. In 2024, Japan’s PMDA approved iRhythm Technologies’ Zio 14-day continuous ECG monitoring system, enhancing cardiac arrhythmia diagnosis compared to traditional Holter monitors. The patch device used AI to provide accurate reports and has been designated for high medical needs in Japan.
Japan Medical Wearables Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2026-2034. Our report has categorized the market based on product, device type, and end user.
Product Insights:
- Patches
- Wristband and Activity Monitors
- Smartwatches
- Others
A detailed breakup and analysis of the market based on the product have also been provided in the report. This includes patches, wristband and activity monitors, smartwatches, and others.
Device Type Insights:
- Diagnostic and Monitoring Medical Devices
- Vital Signs Monitoring Devices
- ECG /Holter Heart Rate Monitors
- Pulse Oximeters
- Blood Pressure Monitors
- Multiparameter Trackers
- Glucose Monitoring Devices
- Sleep Apnea Monitors
- Fetal Monitoring Devices
- Neurological Monitoring Devices
- Vital Signs Monitoring Devices
The report has provided a detailed breakup and analysis of the market based on the device type. This includes diagnostic and monitoring medical devices [vital signs monitoring devices (ECG/Holter heart rate monitors, pulse oximeters, blood pressure monitors, and multiparameter trackers), glucose monitoring devices, sleep apnea monitors, fetal monitoring devices, and neurological monitoring devices].
End User Insights:
Access the comprehensive market breakdown Request Sample
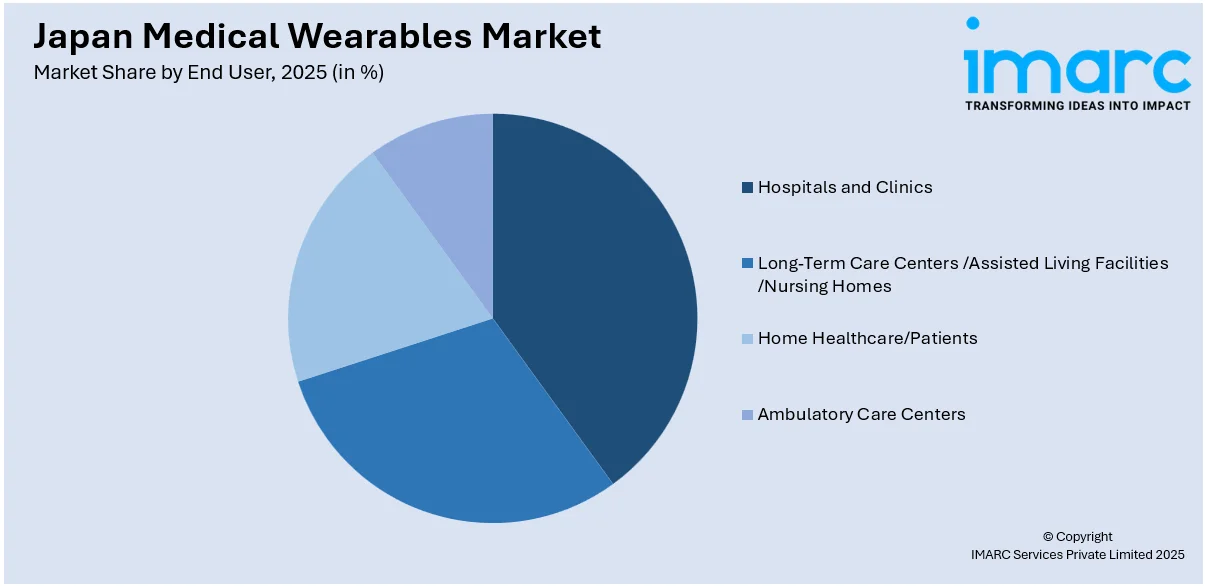
- Hospitals and Clinics
- Long-Term Care Centers /Assisted Living Facilities /Nursing Homes
- Home Healthcare/Patients
- Ambulatory Care Centers
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes hospitals and clinics, long-term care centers /assisted living facilities /nursing homes, home healthcare/patients, and ambulatory care centers.
Regional Insights:
- Kanto Region
- Kansai/Kinki Region
- Central/ Chubu Region
- Kyushu-Okinawa Region
- Tohoku Region
- Chugoku Region
- Hokkaido Region
- Shikoku Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Kanto Region, Kansai/Kinki Region, Central/ Chubu Region, Kyushu-Okinawa Region, Tohoku Region, Chugoku Region, Hokkaido Region, and Shikoku Region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Japan Medical Wearables Market News:
- In March 2025, Sky Labs partnered with OMRON Healthcare to launch its smart ring blood pressure monitor in Japan by year-end, starting with a wellness version ahead of regulatory approval. The ring tracks blood pressure variability, heart rate, activity, and sleep. This collaboration expands OMRON’s reach into non-invasive health monitoring for everyday use.
- In January 2025, MEDIROM MOTHER Labs began providing its recharge-free MOTHER Bracelet® and REMONY remote health monitoring system to TOPPAN Inc.'s Electronics Division in Japan. The system enables 24/7 health tracking without recharging, supporting early detection of health issues. It targets industries like solo-living employee care, elderly care, and night shift monitoring.
Japan Medical Wearables Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered | Patches, Wristband and Activity Monitors, Smartwatches, Others |
| Device Types Covered |
|
| End Users Covered | Hospitals and Clinics, Long-Term Care Centers /Assisted Living Facilities /Nursing Homes, Home Healthcare/Patients, Ambulatory Care Centers |
| Regions Covered | Kanto Region, Kansai/Kinki Region, Central/ Chubu Region, Kyushu-Okinawa Region, Tohoku Region, Chugoku Region, Hokkaido Region, Shikoku Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Japan medical wearables market performed so far and how will it perform in the coming years?
- What is the breakup of the Japan medical wearables market on the basis of product?
- What is the breakup of the Japan medical wearables market on the basis of device type?
- What is the breakup of the Japan medical wearables market on the basis of end user?
- What is the breakup of the Japan medical wearables market on the basis of region?
- What are the various stages in the value chain of the Japan medical wearables market?
- What are the key driving factors and challenges in the Japan medical wearables market?
- What is the structure of the Japan medical wearables market and who are the key players?
- What is the degree of competition in the Japan medical wearables market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Japan medical wearables market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Japan medical wearables market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Japan medical wearables industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












