
Latin America Vaccine Market Size, Share, Trends and Forecast by Technology, Patient Type, Indication, Route of Administration, Product Type, Treatment Type, End User, Distribution Channel, and Country, 2026-2034
Latin America Vaccine Market Overview:
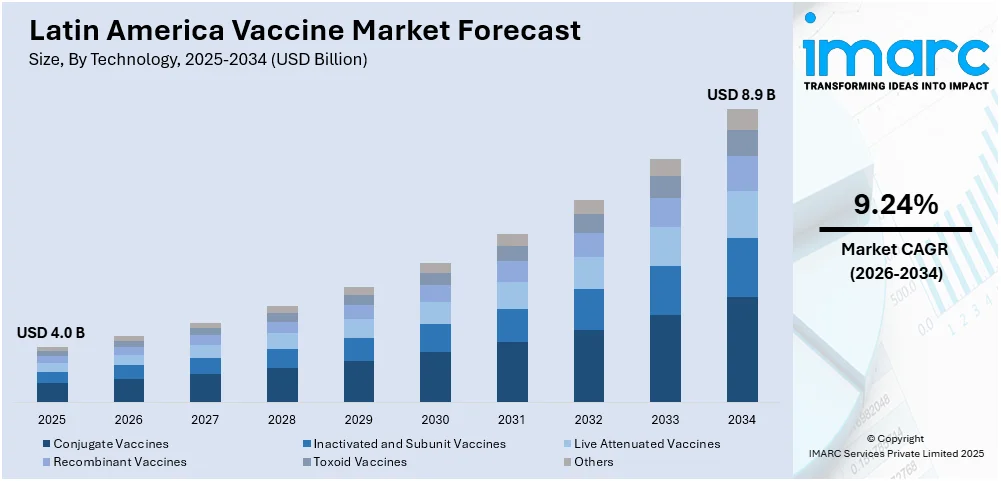
The Latin America vaccine market size reached USD 4.0 Billion in 2025. Looking forward, IMARC Group expects the market to reach USD 8.9 Billion by 2034, exhibiting a growth rate (CAGR) of 9.24% during 2026-2034. The market is expanding due to the increasing government immunization programs, rising public awareness, ongoing advancements in biotechnology, growing local vaccine production, improved cold chain logistics, expanding private-sector investments, and heightened demand for Human Papillomavirus (HPV), dengue, and other preventable disease vaccines.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 4.0 Billion |
| Market Forecast in 2034 | USD 8.9 Billion |
| Market Growth Rate 2026-2034 | 9.24% |
Latin America Vaccine Market Trends:
Growing Government Initiatives and Immunization Programs
The government of Latin America have increased their vaccine programs through enhanced accessibility and coverage of preventable disease vaccines. National vaccination campaigns under the leadership of the Pan American Health Organization (PAHO) and World Health Organization (WHO) strengthen routine immunization efforts against measles, polio, and influenza diseases. For instance, the 22nd annual Vaccination Week in the Americas (VWA) from April 20-27, had over 65 million vaccine doses administered across 34 countries, targeting diseases such as measles, polio, and influenza. The public-private sector partnerships also encompass research and production of vaccines and their distribution networks. As part of their support efforts governments throughout the world provide their disadvantaged populations with free access to vaccines. Furthermore, the increasing investments in healthcare infrastructure and cold chain logistics systems create advanced storage and distribution systems for vaccines. These combined activities have driven vaccination coverage to increase and minimize disease severity alongside creating a rising demand for new vaccine products, thus expanding the Latin America vaccine market share.
To get more information on this market Request Sample
Expansion of HPV and Dengue Vaccination Programs
The acceptance of human papillomavirus (HPV) and dengue vaccines is rising throughout Latin America because residents have become more aware of these health threats. In line with this, national governments now incorporate HPV vaccines into their official vaccination programs to decrease cervical cancer occurrence mostly in adolescent girls. Moreover, the healthcare challenge from dengue disease drives Brazil Mexico, and Colombia to implement expanded vaccination programs. For example, in 2024, Americas reported a record 12.6 million suspected dengue cases in 2024, nearly tripling the previous year's figures, with Brazil alone accounting for over 10 million cases. Besides this, the release of vaccines such as Qdenga and Dengvaxia, along with the ongoing research for future vaccines are anticipated to provide better results. Furthermore, the rising prevalence of vector-borne diseases interlinked with climate change has increased the adoption of dengue vaccines. Apart from this, pharmaceutical businesses have increased their production levels as they need to align with the regional market requirements, thereby enhancing the Latin America vaccine market outlook.
Technological Advancements in Vaccine Development and Production
Ongoing advancements in biotechnology are transforming vaccine development and production across Latin America. Governments throughout the region devote resources to creating new vaccine approaches based on recombinant protein and viral vector tech to improve both product performance and duration of protection. Additionally, regional manufacturers advance local vaccine production to establish self-sufficiency while they decrease their dependence on imported vaccines. For instance, Argentina’s Sinergium Biotech launched a project in July 2024 to accelerate the development of mRNA vaccines against human avian influenza (H5N1), collaborating with the World Health Organization (WHO) and the Medicines Patent Pool (MPP) to strengthen regional vaccine self-reliance. Also, the governments of Brazil, Argentina, and Mexico together with international partnerships stand behind the local development of vaccines through available public support. Through digital health technologies, the immunization programs receive better vaccine tracking capabilities and enhanced distribution efficiency as well as improved patient compliance. Moreover, the integration of artificial intelligence (AI) systems with big data analysis tools enables faster vaccine creation along with better disease monitoring capabilities. These continuous advancements are driving innovation and ensuring more resilience in Latin America vaccine market growth.
Latin America Vaccine Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country level for 2026-2034. Our report has categorized the market based on technology, patient type, indication, route by administration, product type, treatment type, end user, and distribution channel.
Technology Insights:
- Conjugate Vaccines
- Inactivated and Subunit Vaccines
- Live Attenuated Vaccines
- Recombinant Vaccines
- Toxoid Vaccines
- Others
The report has provided a detailed breakup and analysis of the market based on the technology. This includes conjugate vaccines, inactivated and subunit vaccines, live attenuated vaccines, recombinant vaccines, toxoid vaccines, and others.
Patient Type Insights:
- Pediatric
- Adult
A detailed breakup and analysis of the market based on the patient type have also been provided in the report. This includes pediatric and adult.
Indication Insights:
- Bacterial Diseases
- Meningococcal Disease
- Pneumococcal Disease
- Diphtheria/Tetanus/Pertussis (DPT)
- Tuberculosis
- Haemophilus Influenzae (Hib)
- Typhoid
- Others
- Viral Diseases
- Hepatitis
- Influenza
- Human Papillomavirus (HPV)
- Measles/Mumps/Rubella (MMR)
- Rotavirus
- Herpes Zoster
- Varicella
- Latinamericaese Encephalitis
- Rubella
- Polio
- Rabies
- Dengue
- Others
The report has provided a detailed breakup and analysis of the market based on the indication. This includes bacterial diseases (meningococcal disease, pneumococcal disease, diphtheria/tetanus/pertussis (DPT), tuberculosis, haemophilus influenzae (Hib), typhoid, and others), and viral diseases (hepatitis, influenza, human papillomavirus (HPV), measles/mumps/rubella (MMR), rotavirus, herpes zoster, varicella, latinamericaese encephalitis, rubella, polio, rabies, dengue, and others).
Route of Administration Insights:
- Intramuscular and Subcutaneous Administration
- Oral Administration
- Others
A detailed breakup and analysis of the market based on the route of administration have also been provided in the report. This includes intramuscular and subcutaneous administration, oral administration, and others.
Product Type Insights:
- Multivalent Vaccine
- Monovalent Vaccine
The report has provided a detailed breakup and analysis of the market based on the product type. This includes multivalent vaccine and monovalent vaccine.
Treatment Type Insights:
- Preventive Vaccine
- Therapeutic Vaccine
A detailed breakup and analysis of the market based on the treatment type have also been provided in the report. This includes preventive vaccine and therapeutic vaccine.
End User Insights:
Access the Comprehensive Market Breakdown Request Sample
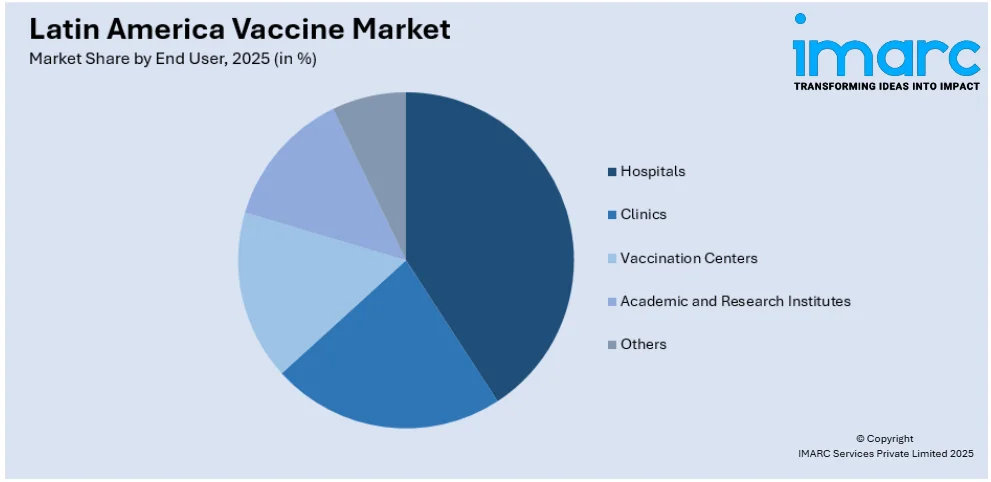
- Hospitals
- Clinics
- Vaccination Centers
- Academic and Research Institutes
- Others
The report has provided a detailed breakup and analysis of the market based on the end user. This includes hospitals, clinics, vaccination centers, academic and research institutes, and others.
Distribution Channel Insights:
- Hospital Pharmacies
- Retail Pharmacies
- Institutional Sales
- Others
A detailed breakup and analysis of the market based on the distribution channel have also been provided in the report. This includes hospital pharmacies, retail pharmacies, institutional sales, and others.
Country Insights:
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Peru
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Brazil, Mexico, Argentina, Colombia, Chile, Peru, and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Latin America Vaccine Market News:
- In January 2025, the Pan American Health Organization (PAHO), Argentina, Pfizer, and Sinergium Biotech announced a collaboration to produce the 20-valent pneumococcal conjugate vaccine (PCV20) locally. This initiative aims to enhance vaccine accessibility across Latin America and the Caribbean, with the first doses expected by 2026.
- In September 2024, Brazil's Beep Saude, a mobile vaccination service, raised 100 million reais ($17.64 million) from a U.S.-based climate-focused firm. The investment will support Beep's expansion and enhance its logistics to meet the growing demand for vaccines, especially those related to mosquito-borne diseases.
Latin America Vaccine Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Technologies Covered | Conjugate Vaccines, Inactivated and Subunit Vaccines, Live Attenuated Vaccines, Recombinant Vaccines, Toxoid Vaccines, Others |
| Patient Types Covered | Pediatric, Adult |
| Indications Covered |
|
| Route of Administrations Covered | Intramuscular and Subcutaneous Administration, Oral Administration, Others |
| Product Types Covered | Multivalent Vaccine, Monovalent Vaccine |
| Treatment Types Covered | Preventive Vaccine, Therapeutic Vaccine |
| End Users Covered | Hospitals, Clinics, Vaccination Centers, Academic and Research Institutes, Others |
| Distribution Channels Covered | Hospital Pharmacies, Retail Pharmacies, Institutional Sales, Others |
| Countries Covered | Brazil, Mexico, Argentina, Colombia, Chile, Peru, Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Latin America vaccine market performed so far and how will it perform in the coming years?
- What is the breakup of the Latin America vaccine market on the basis of technology?
- What is the breakup of the Latin America vaccine market on the basis of patient type?
- What is the breakup of the Latin America vaccine market on the basis of indication?
- What is the breakup of the Latin America vaccine market on the basis of route of administration?
- What is the breakup of the Latin America vaccine market on the basis of product type?
- What is the breakup of the Latin America vaccine market on the basis of treatment type?
- What is the breakup of the Latin America vaccine market on the basis of end user?
- What is the breakup of the Latin America vaccine market on the basis of distribution channel?
- What is the breakup of the Latin America vaccine market on the basis of country?
- What are the various stages in the value chain of the Latin America vaccine market?
- What are the key driving factors and challenges in the Latin America vaccine?
- What is the structure of the Latin America vaccine market and who are the key players?
- What is the degree of competition in the Latin America vaccine market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Latin America vaccine market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Latin America vaccine market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Latin America vaccine industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












