
Mexico Pharmacovigilance Market Size, Share, Trends and Forecast by Service Provider, Product Life Cycle, Type, Process Flow, Therapeutic Area, End Use, and Region, 2025-2033
Mexico Pharmacovigilance Market Overview:
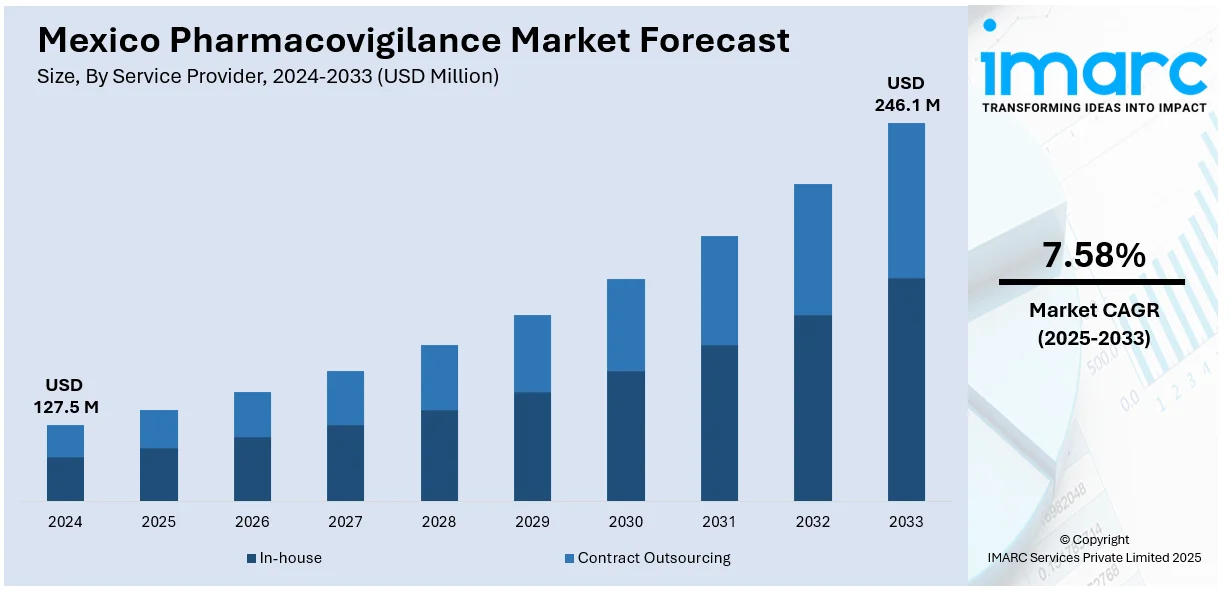
The Mexico pharmacovigilance market size reached USD 127.5 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 246.1 Million by 2033, exhibiting a growth rate (CAGR) of 7.58% during 2025-2033. The market is driven by growing regulatory enforcement, increasing adverse drug reaction (ADR) reporting, expanding pharmaceutical industry, rising chronic disease prevalence, surging clinical trials, digital health integration, and heightened public awareness of drug safety.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 127.5 Million |
| Market Forecast in 2033 | USD 246.1 Million |
| Market Growth Rate 2025-2033 | 7.58% |
Mexico Pharmacovigilance Market Trends:
Strengthening Regulatory Frameworks and Compliance
The Mexico pharmacovigilance market share is expanding, due to the COFEPRIS and the National Pharmacovigilance Centre, as they have strengthened their regulatory systems. In line with this, the pharmaceutical sector follows requirements from COFEPRIS and the National Pharmacovigilance Centre to develop strict pharmacovigilance programs, which need continuous ADR reporting and risk management planning. Moreover, Mexico now applies real-time reporting technology because of regulatory updates that bring its standards closer to international pharmaceutical safety requirements. For example, in March 2024, COFEPRIS issued new Good Manufacturing Practice (GMP) guidelines, accepting certifications from regulatory agencies in Brazil, Argentina, Canada, and the U.S. This alignment with international standards aims to improve drug quality assurance and bolster pharmacovigilance practices. Besides this, the increased regulatory pressure makes pharmaceutical companies allocate funds to buy specialized resources and training programs. Furthermore, the post-market surveillance system, along with reporting efficiency, has significantly improved. Apart from this, the updated regulations are strengthening drug safety and creating confidence among stakeholders, which is increasing the investment in the pharmacovigilance system across the region.
Integration of Advanced Technologies in Pharmacovigilance
The Mexico pharmacovigilance market outlook is experiencing substantial changes because of the implementation of advanced technologies, including artificial intelligence (AI), machine learning (ML), and big data analytics. The combination of these technological tools improves ADR assessment by processing cases automatically and strengthening signal discovery abilities and boosting the decision-making process. Concurrently, the pharmaceutical industry now implements digital systems that obtain real-time information through electronic health records, along with mobile application data. For instance, in 2023, Mexico's regulatory authority, COFEPRIS, updated its Digital Research and Clinical Trials Platform (DIGIPRiS) to streamline the electronic submission and review of research protocols. This advancement aims to expedite approvals and improve the efficiency of pharmacovigilance activities by facilitating real-time data access and monitoring. In confluence with this, these changes enable precise drug risk assessment while fueling the detection of potential adverse events ahead of time, which minimizes dependence on traditional manual methods. Additionally, technology integration in pharmacovigilance operations leads to more operational effectiveness and improved patient safety results while streamlining modern pharmacovigilance processes. As a result, this trend is driving the Mexico pharmacovigilance market growth and positioning the region as a leading player in worldwide pharmacovigilance operations.
Mexico Pharmacovigilance Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the region level for 2025-2033. Our report has categorized the market based on service provider, product life cycle, type, process flow, therapeutic area and end use.
Service Provider Insights:
- In-house
- Contract Outsourcing
The report has provided a detailed breakup and analysis of the market based on the service provider. This includes in-house and contract outsourcing.
Product Life Cycle Insights:
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
A detailed breakup and analysis of the market based on the product life cycle have also been provided in the report. This includes pre-clinical, phase I, phase II, phase III, and phase IV.
Type Insights:
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
The report has provided a detailed breakup and analysis of the market based on the type. This includes spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring, and EHR mining.
Process Flow Insights:
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing and Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review and Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
A detailed breakup and analysis of the market based on the process flow have also been provided in the report. This includes case data management (case logging, case data analysis, and medical reviewing and reporting), signal detection (adverse event logging, adverse event analysis, and adverse event review and reporting), and risk management system (risk evaluation system and risk mitigation system).
Therapeutic Area Insights:
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
The report has provided a detailed breakup and analysis of the market based on the therapeutic area. This includes oncology, neurology, cardiology, respiratory systems, and others.
End Use Insights:
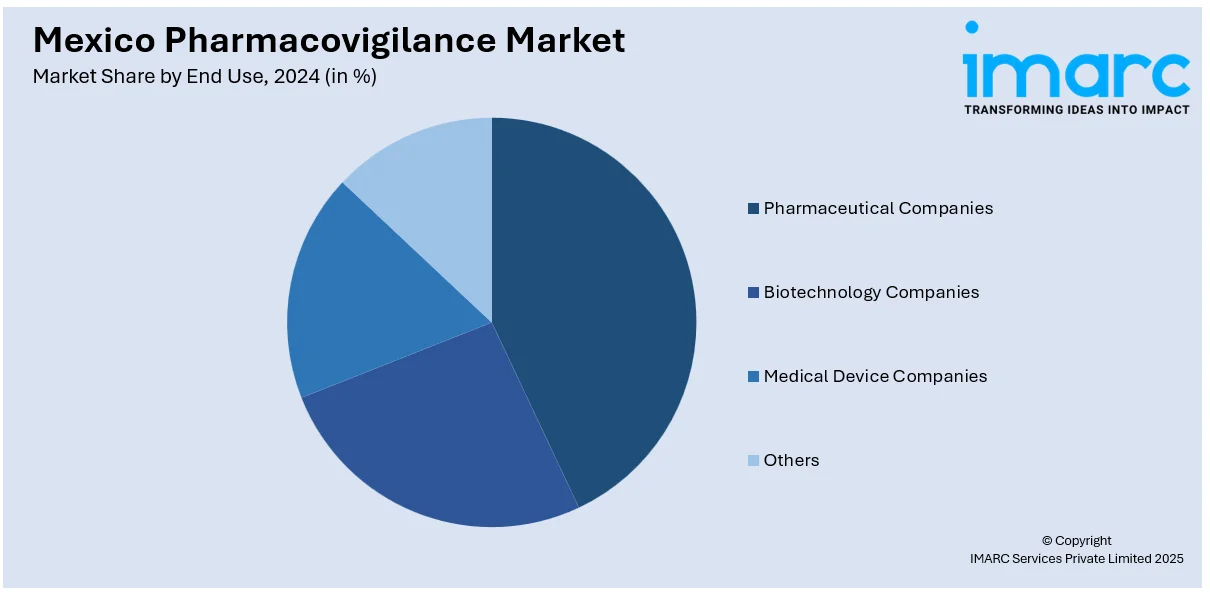
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Companies
- Others
A detailed breakup and analysis of the market based on the end use have also been provided in the report. This includes pharmaceutical companies, biotechnology companies, medical device companies, and others.
Regional Insights:
- Northern Mexico
- Central Mexico
- Southern Mexico
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Northern Mexico, Central Mexico, Southern Mexico, and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Mexico Pharmacovigilance Market News:
- In April 2025, the regulatory authority, COFEPRIS, strengthened its New Molecules Committee to speed up the approval of novel pharmaceuticals. By expediting drug approvals, this initiative facilitates quicker market entry for new medications, necessitating robust pharmacovigilance to ensure ongoing drug safety monitoring.
- In May 2024, M8 Pharmaceuticals, an Acino company, signed an exclusive licensing agreement with Supernus Pharmaceuticals to seek regulatory approval and commercialize Qelbree® (Viloxazine XR) in Latin America. This partnership introduces innovative treatments to the Latin American market, enhancing therapeutic options and driving pharmacovigilance activities to monitor the drug's safety and efficacy.
Mexico Pharmacovigilance Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Service Providers Covered | In-house, Contract Outsourcing |
| Product Life Cycles Covered | Pre-clinical, Phase I, Phase II, Phase III, Phase IV |
| Types Covered | Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, EHR Mining |
| Process Flows Covered |
|
| Therapeutic Areas Covered | Oncology, Neurology, Cardiology, Respiratory Systems, Others |
| End Uses Covered | Pharmaceutical Companies, Biotechnology Companies, Medical Device Companies, Others |
| Regions Covered | Northern Mexico, Central Mexico, Southern Mexico, Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Mexico pharmacovigilance market performed so far and how will it perform in the coming years?
- What is the breakup of the Mexico pharmacovigilance market on the basis of service provider?
- What is the breakup of the Mexico pharmacovigilance market on the basis of product life cycle?
- What is the breakup of the Mexico pharmacovigilance market on the basis of type?
- What is the breakup of the Mexico pharmacovigilance market on the basis of process flow?
- What is the breakup of the Mexico pharmacovigilance market on the basis of therapeutic area?
- What is the breakup of the Mexico pharmacovigilance market on the basis of end use?
- What is the breakup of the Mexico pharmacovigilance market on the basis of region?
- What are the various stages in the value chain of the Mexico pharmacovigilance market?
- What are the key driving factors and challenges in the Mexico pharmacovigilance?
- What is the structure of the Mexico pharmacovigilance market and who are the key players?
- What is the degree of competition in the Mexico pharmacovigilance market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Mexico pharmacovigilance market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Mexico pharmacovigilance market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Mexico pharmacovigilance industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












