Plaque Psoriasis Market Size to Reach USD 32.3 Billion by 2035, Impelled by Advancements in Early Detection
Plaque Psoriasis Market Outlook 2025-2035:
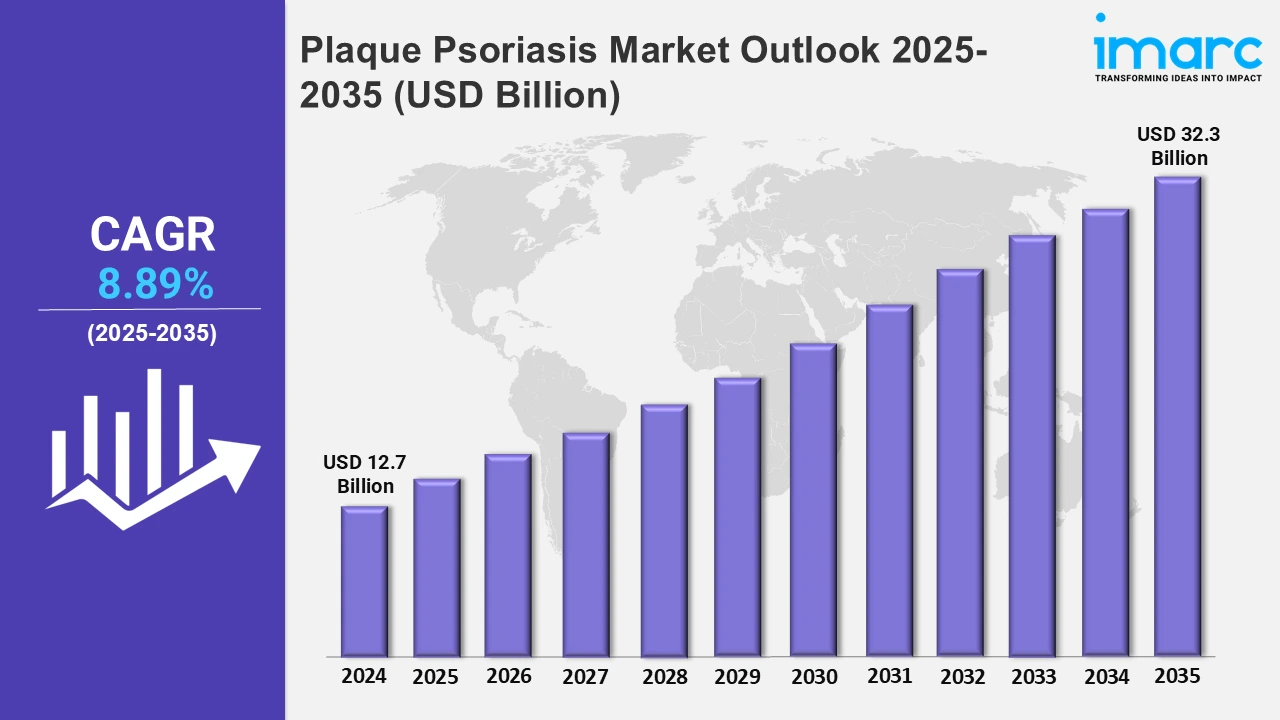
The 7 major plaque psoriasis market reached a value of USD 12.7 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 32.3 Billion by 2035, exhibiting a growth rate (CAGR) of 8.89% during 2025-2035. The market is driven by various key players who are making extensive investments in developing novel therapies to manage cytokines and other immune system proteins that promote skin inflammation and the formation of psoriatic plaques. Additionally, the development of targeted therapies and innovative treatments is further propelling the market growth.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Plaque Psoriasis Market
Advances in the early stages of detection and diagnostic technology have led to the enormous growth of the plaque psoriasis market. The development of high resolution dermoscopy and OCT, has been one of the major technological advances in diagnostic imaging. Amongst these, non-invasive imaging means provide the dermatologist with a way of visualizing the layers of skin in such a manner that may differentiate plaque psoriasis from inflammatory skin diseases like eczema or even fungal infections. Moreover, real-time cellular level imaging through CLSM also boosts up the principles of diagnostic accuracy. Biomarker research is also changing the face of early diagnosis and disease monitoring. Several specific genetic markers, cytokines, and inflammatory proteins have been identified in the studies. Among these markers, genetic markers and cytokines/inflammatory proteins like IL-17, IL-23, and TNF-α have been found to correlate with disease activity and treatment responses. Biomarkers in blood tests and skin biopsies are increasingly developed for predicting the severity of disease, progression, and effectiveness to targeted treatments. Integration of AI and machine learning algorithms with teledermatology and digital health platforms allows for easier detection at an earlier stage. Image processing of the skin lesion may be done and preliminary diagnosis can be made by an AI-based application. This ensures early teleconsultation and referrals from specialists by the patients. All these advancements thereby improve the quality of care concerning the early accurate diagnosis of plaque psoriasis and are hence driving the expansion of the plaque psoriasis market. Future expansions of the market for plaque psoriasis will be supported through further research work in developing newer diagnostic aids such that proper diseases can be monitored and thus maintain improved lives of patients in the long term.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The plaque psoriasis market is growing rapidly with developments in novel therapy and pharmacologic treatments that produce improved efficacy with longer-lasting benefits and fewer adverse effects. Innovative biologic treatments, small molecule drugs, and novel treatment modes are changing disease management and helping drive the plaque psoriasis drug market. Because they target selected inflammatory pathways without suppression of immunity, biological therapies have replaced traditional treatments. There are newer generation biologics including risankizumab (Skyrizi), guselkumab (Tremfya), and bimekizumab (Bimzelx). All these molecules selectively inhibit the activities of either IL-23 or IL-17, conferring long-lasting remission while generating fewer systemic side effects in contrast to earlier generations. Also, patients gain greater access through biosimilars, which are affordable substitutes for traditional biologics such as adalimumab (Humira) and etanercept (Enbrel). In addition to biologics, oral small-molecule drugs, like deucravacitinib (Sotyktu), a TYK2 inhibitor, and apremilast (Otezla) a PDE4 inhibitor, offer simple, injectable treatments to all patients suffering from psoriasis, including moderate or worse ones. Targeted therapies are bringing safer long-term use with few risks of immunosuppression. Innovative topical treatments and delivery technologies, such as nanoparticle formulations and gene therapy, are transforming the landscape. New options are emerging with rapid evolution from JAK inhibitors and treatments based on the microbiome, which also opens the way for treatment in refractory cases. Ongoing associated FDA approval and future investment into research further grows the market of plaque psoriasis as improved options for treatments, better outcomes, and improved quality of life for the patient who suffers with the chronic condition are needed.
Marketed Therapies in Plaque Psoriasis Market
Enbrel (Etanercept): Amgen
Enbrel (Etanercept) is a tumor necrosis factor (TNF) inhibitor. It is used to treat plaque psoriasis by blocking excessive inflammation. It is a soluble TNF receptor fusion protein that binds to TNF-α, a key cytokine involved in the inflammatory response of psoriasis. By preventing TNF-α from interacting with its receptors on immune cells, Enbrel reduces inflammation, keratinocyte proliferation, and plaque formation. This leads to clearer skin, reduced scaling, and symptom relief.
Duobrii (Tazarotene/Halobetasol): Ortho Dermatologics
Duobrii is a combination of tazarotene (a retinoid) and halobetasol propionate (a corticosteroid). It treats plaque psoriasis by targeting both keratinocyte proliferation and inflammation. Tazarotene inhibits excessive skin cell growth by binding to retinoid receptors. Halobetasol suppresses inflammation by binding to glucocorticoid receptors, thereby effectively reducing the rapid skin cell turnover characteristic of psoriasis. As a result, both components help to reduce the appearance of psoriatic plaques.
Siliq (Brodalumab): Ortho Dermatologics
Siliq (brodalumab) is used to treat moderate to severe plaque psoriasis in adult patients who have not responded to previous systemic treatments or phototherapy. Brodalumab is a monoclonal IgG2 antibody that binds to human IL-17RA and inhibits its interactions with other cytokines, including IL-17A, IL-17F, IL-17C, IL-17A/F heterodimer, and IL-25. IL-17RA, a protein produced on the cell surface, is a necessary component of receptor complexes used by many IL-17 family cytokines. Blocking IL17RA decreases IL-17 cytokine-induced responses, such as the release of pro-inflammatory cytokines and chemokines.
Emerging Therapies in Plaque Psoriasis Market
Izokibep: Affibody
Izokibep is a bispecific interleukin (IL) 17A inhibitor. It is being developed by Affibody for the treatment of moderate to severe psoriasis. Izokibep is designed to inhibit IL-17A with high potency via tight binding affinity. Its potential for robust tissue penetration due to its small molecular size (about one-tenth the size of a monoclonal antibody) and an albumin binding domain result in improved pharmacokinetic (PK) properties. Clinical study data support the concept that izokibep's unique qualities may give clinically substantial and differentiated advantages to patients, such as the remission of the illness symptoms.
ESK-001: Alumis
ESK001 is an investigational TYK2 (tyrosine kinase 2) inhibitor developed by Alumis for the treatment of plaque psoriasis. TYK2 is a key enzyme involved in the JAK-STAT signaling pathway, mediating inflammatory responses driven by IL-23, IL-12, and type I interferons. By selectively inhibiting TYK2, ESK001 reduces the activation of inflammatory cytokines that contribute to keratinocyte hyperproliferation and immune system overactivity in psoriasis. This targeted approach lowers inflammation, minimizes skin plaque formation, and improves disease symptoms while potentially offering better safety and tolerability.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Izokibep | Affibody | IL17A protein inhibitors | Intravenous, subcutaneous |
| ESK-001 | Alumis | TYK2 kinase inhibitors | Oral |
Detailed list of emerging therapies in Plaque Psoriasis is provided in the final report…
Leading Companies in the Plaque Psoriasis Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global plaque psoriasis market, several leading companies are at the forefront of developing integrated platforms to enhance the management of plaque psoriasis. Some of the major players include Amgen, Ortho Dermatologics, and Sun Pharmaceutical Industries. These companies are driving innovation in the plaque psoriasis market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for the illness.
In May 2023, Sun Pharma announced that the Chinese National Medical Products Administration (NMPA) approved its new drug application for tildrakizumab injection used in the treatment of plaque psoriasis.
Key Players in Plaque Psoriasis Market:
The key players in the Plaque Psoriasis market who are in different phases of developing different therapies are Amgen, Ortho Dermatologics, Sun Pharmaceutical Industries, Arcutis Biotherapeutics, Alumis, Affibody, Janssen, Takeda, MetrioPharm, and Others.

Regional Analysis:
The major markets for plaque psoriasis include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for plaque psoriasis while also representing the biggest market for its treatment. This can be attributed to the rising incidence of autoimmune disorders, genetic predisposition, and environmental triggers, along with numerous lifestyle factors such as stress, smoking, obesity, and alcohol consumption that contribute to the increasing number of psoriasis cases.
Development and adoption of biologic therapies have also been major drivers of the plaque psoriasis market in the United States. This is because, with the entry of biologics into this field of management, treatment approaches for psoriasis have significantly shifted, promising more efficacy, prolonged remission, and reduced systemic side effects of therapy.
In addition, there are small-molecule drugs that include TYK2 inhibitors, such as Sotyktu and ESK001, and PDE4 inhibitors, including Otezla. Increasingly, oral therapies are available to patients who prefer them over injectable biologics, and the demand for targeted, non-immunosuppressive treatments is driving adoption and market expansion.
Recent Developments in Plaque Psoriasis Market:
- In March 2024, Alumis Inc. reported encouraging clinical results from a Phase 2 clinical trial of ESK-001, a highly selective allosteric tyrosine kinase 2 (TYK2) inhibitor, for the treatment of moderate-to-severe plaque psoriasis. The trial fulfilled its primary aim, the proportion of patients attaining a 75% improvement in the Psoriasis Area and Severity Score (PASI 75) at week 12 compared to placebo, as well as significant secondary efficacy endpoints across all clinically relevant doses evaluated.
- In March 2024, Johnson & Johnson released the first results from FRONTIER 2, a long-term extension of the Phase 2b FRONTIER 1 clinical trial testing JNJ-2113, the first and only investigational targeted oral peptide designed to inhibit the IL-23 receptor. IL-23 is essential for pathogenic T-cell activation in moderate-to-severe plaque psoriasis (PsO), as well as for the inflammatory response in PsO and other IL-23-mediated illnesses in dermatology, rheumatology, and gastroenterology.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the plaque psoriasis market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the plaque psoriasis market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current plaque psoriasis marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












