
Platelet Aggregation Devices Market Report by Product (Systems, Reagents, Consumables and Accessories), Type (Four Channel, Dual Channel, Eight Channel), Application (Clinical Applications, Research Applications, Cardiovascular Applications, Orthopedic Applications, and Others), End User (Hospitals, Diagnostic Laboratories, Research and Academic Institutes, and Others), and Region 2026-2034
Market Overview:
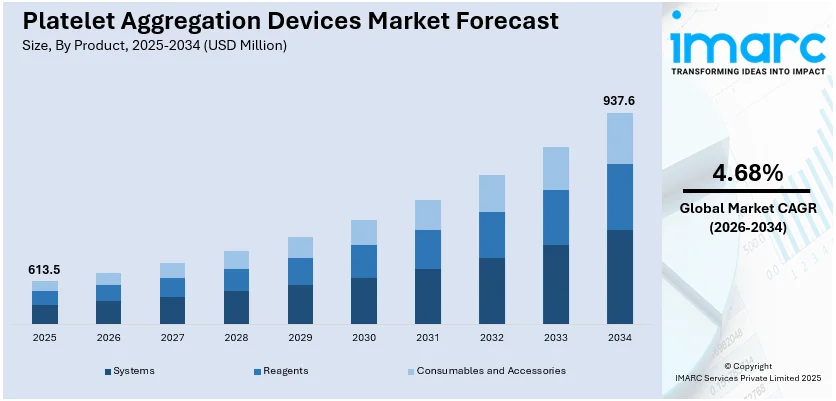
The global platelet aggregation devices market size reached USD 613.5 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 937.6 Million by 2034, exhibiting a growth rate (CAGR) of 4.68% during 2026-2034.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025
|
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 613.5 Million |
| Market Forecast in 2034 | USD 937.6 Million |
| Market Growth Rate (2026-2034) | 4.68% |
Platelet aggregation devices comprise adenosine-diphosphate (ADP), thrombin, and ristocetin-induced platelet aggregation (RIPA). These devices are used to diagnose patients with inherited and acquired platelet function disorders, such as excessive bleeding, arterial thrombotic, stroke, and myocardial infarction. They assist in transfusing medicines, pre-surgery tests, and clinical settings to monitor the response of antiplatelet therapies and assess perioperative bleeding risk. At present, due to the launch of new potential biomarkers and technologies, platelet aggregation devices are finding extensive application in personalizing antiplatelet drugs and monitoring the efficacy of various types of pro-hemostatic therapies around the world.
To get more information on this market Request Sample
Platelet Aggregation Devices Market Trends:
The rising instances of platelet aggregation among patients diagnosed with coronavirus disease (COVID-19) are strengthening the growth of the market. Moreover, due to the growing prevalence of colon cancer, cardiovascular diseases, Kawasaki condition, acute coronary syndrome (ACS), acute ischemic stroke, and acute rheumatic disorders across the globe, there is an increase in the risk of bleeding. This acts as another key factor impelling the growth of the platelet aggregation devices market for clinical management of platelet function disorders. In addition, a significant rise in the aging population suffering from chronic diseases is contributing to the market growth. Apart from this, leading manufacturers are launching novel products to expand their product portfolio and retain a competitive edge in the market. They are also focusing on developing new and simpler point-of-care (POC) diagnostic devices. These devices rely on the latest methodologies for helping in the rapid evaluation of inherited and acquired bleeding disorders and antiplatelet therapies. This, coupled with the increasing need for POC diagnostic devices to fulfill the healthcare demand of the large population, is projected to drive the market.
Key Market Segmentation:
IMARC Group provides an analysis of the key trends in each sub-segment of the global platelet aggregation devices market report, along with forecasts at the global, regional and country level from 2026-2034. Our report has categorized the market based on product, type, application and end user.
Breakup by Product:
- Systems
- Reagents
- Consumables and Accessories
Breakup by Type:
- Four Channel
- Dual Channel
- Eight Channel
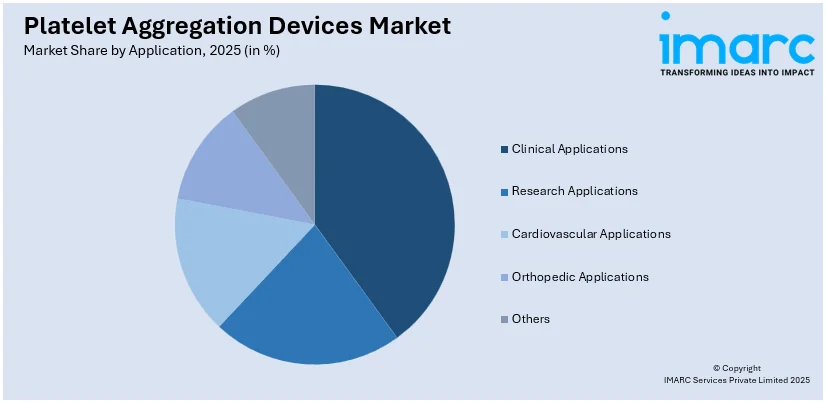
Breakup by Application:
Access the comprehensive market breakdown Request Sample
- Clinical Applications
- Research Applications
- Cardiovascular Applications
- Orthopedic Applications
- Others
Breakup by End User:
- Hospitals
- Diagnostic Laboratories
- Research and Academic Institutes
- Others
Breakup by Region:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
Competitive Landscape:
The competitive landscape of the industry has also been examined along with the profiles of the key players being Aggredyne Inc., Chrono-Log Corporation, F. Hoffmann-La Roche AG, Grifols S.A., Hart Biologicals Ltd., Helena Laboratories Corporation, LAbor BioMedical Technologies GmbH, Matis Medical Inc., Sentinel Ch. S.P.A., Siemens AG, Sienco Inc., Sysmex Corporation and WerfenLife SA.
Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Segment Coverage | Product, Type, Application, End User, Region |
| Region Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Aggredyne Inc., Chrono-Log Corporation, F. Hoffmann-La Roche AG, Grifols S.A., Hart Biologicals Ltd., Helena Laboratories Corporation, LAbor BioMedical Technologies GmbH, Matis Medical Inc., Sentinel Ch. S.P.A., Siemens AG, Sienco Inc., Sysmex Corporation and WerfenLife SA |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report
The global platelet aggregation devices market was valued at USD 613.5 Million in 2025.
We expect the global platelet aggregation devices market to exhibit a CAGR of 4.68% during 2026-2034.
The sudden outbreak of the COVID-19 pandemic has led to the increasing demand for platelet aggregation devices for treating platelet aggregation disorder, that is caused as a side-effect among the coronavirus-infection patients.
The rising prevalence of various chronic diseases, such as cardiovascular disorders, colon cancer, Kawasaki condition, etc., along with the growing adoption of platelet aggregation devices for clinical management of platelet function disorders, is primarily driving the global platelet aggregation devices market.
Based on the product, the global platelet aggregation devices market has been divided into systems, reagents, and consumables and accessories. Among these, reagents currently exhibit a clear dominance in the market.
Based on the type, the global platelet aggregation devices market can be categorized into four channel, dual channel, and eight channel. Currently, four channel accounts for the majority of the global market share.
Based on the application, the global platelet aggregation devices market has been segregated into clinical applications, research applications, cardiovascular applications, orthopedic applications, and others, where clinical applications currently hold the largest market share.
Based on the end user, the global platelet aggregation devices market can be bifurcated into hospitals, diagnostic laboratories, research and academic institutes, and others. Currently, hospitals exhibit a clear dominance in the market.
On a regional level, the market has been classified into North America, Asia Pacific, Europe, Latin America, and Middle East and Africa, where North America currently dominates the global market.
Some of the major players in the global platelet aggregation devices market include Aggredyne Inc., Chrono-Log Corporation, F. Hoffmann-La Roche AG, Grifols S.A., Hart Biologicals Ltd., Helena Laboratories Corporation, LAbor BioMedical Technologies GmbH, Matis Medical Inc., Sentinel Ch. S.P.A., Siemens AG, Sienco Inc., Sysmex Corporation, and WerfenLife SA.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












