
Saudi Arabia Cell and Gene Therapy Market Size, Share, Trends and Forecast by Therapy Type, Indication, Delivery Mode, End User, and Region, 2026-2034
Saudi Arabia Cell and Gene Therapy Market Overview:
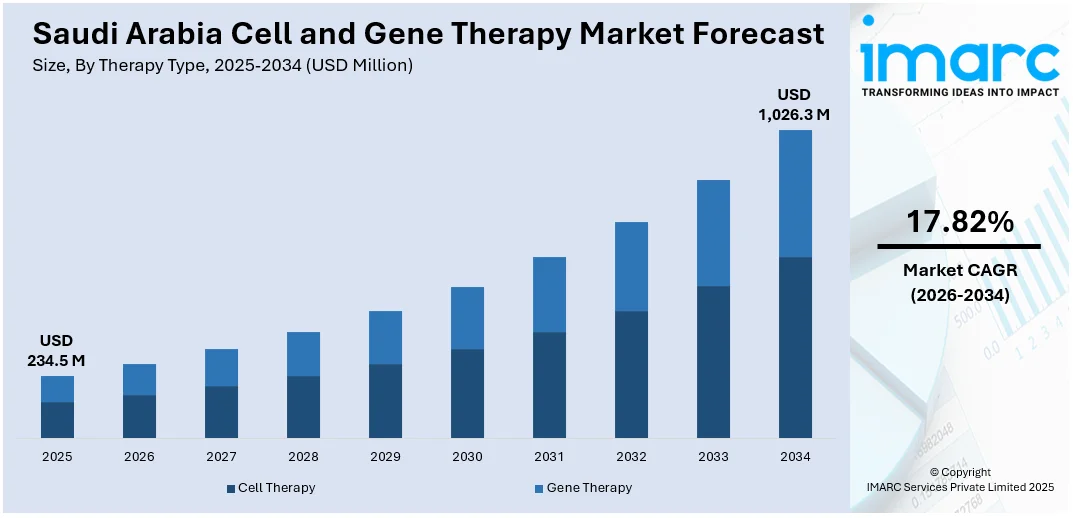
The Saudi Arabia cell and gene therapy market size reached USD 234.5 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 1,026.3 Million by 2034, exhibiting a growth rate (CAGR) of 17.82% during 2026-2034. The market is advancing through localized manufacturing, cost-reduction strategies, and infrastructure expansion. Driven by Vision 2030, the country is focusing on self-reliance, affordability, and innovation to improve patient access and build a strong regional position in advanced therapeutics.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 234.5 Million |
| Market Forecast in 2034 | USD 1,026.3 Million |
| Market Growth Rate 2026-2034 | 17.82% |
Saudi Arabia Cell and Gene Therapy Market Trends:
National Push for Therapy Localization
A strong shift toward localizing advanced therapeutic solutions is significantly shaping Saudi Arabia’s cell and gene therapy market. This focus on domestic capability building aims to address long-standing reliance on external suppliers, reduce logistical bottlenecks, and improve the availability of life-saving treatments for genetic and rare diseases. By localizing manufacturing and clinical infrastructure, the country is strengthening its healthcare ecosystem and enabling faster delivery of therapies to patients in need. In July 2024, Boston Oncology and King Fahd Medical City (KFMC) signed a Letter of Intent to collaborate on localizing cell and gene therapy within Saudi Arabia. This agreement emphasized building national research capacity, expanding clinical trial frameworks, and facilitating the in-country delivery of advanced medical technologies. The partnership also aligned closely with Vision 2030’s focus on healthcare transformation and local industry development. As a result, this trend supports not only improved access to care but also the establishment of a sustainable biotechnology environment. By involving local institutions in advanced therapy development, Saudi Arabia is laying the groundwork for long-term medical innovation. The localization of therapies is expected to drive regional leadership, reduce treatment wait times, improve patient outcomes, and provide a foundation for a knowledge-based economy driven by medical research and technology.
To get more information on this market Request Sample
Emphasis on Affordability and Scale
Saudi Arabia’s growing cell and gene therapy sector is now prioritizing affordability and production scalability as core drivers of market development. The high costs traditionally associated with such treatments have limited access for many patients, especially in regions where international sourcing creates additional financial and logistical barriers. To overcome this, leading healthcare institutions are investing in domestic manufacturing strategies that lower costs and boost production efficiency. In October 2024, King Faisal Specialist Hospital & Research Centre (KFSHRC) began local manufacturing of CAR-T cells, achieving a dramatic 80% reduction in therapy costs down from SAR 1.3 Million per patient. With plans to scale up to 100 gene therapies annually, the move positioned KFSHRC as a major contributor to the Kingdom’s healthcare transformation. This development is aligned with Saudi Arabia’s National Biotechnology Strategy and Vision 2030, aiming to create a diversified and innovation-led economy. The focus on scale ensures that a greater number of patients can benefit from therapies that were once inaccessible due to cost and complexity. Additionally, producing these therapies locally enhances quality control, accelerates turnaround times, and minimizes treatment delays. This approach is creating a sustainable and scalable model for delivering next-generation treatments across the region.
Saudi Arabia Cell and Gene Therapy Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the region/country level for 2026-2034. Our report has categorized the market based on therapy type, indication, delivery mode, and end user.
Therapy Type Insights:
- Cell Therapy
- Stem Cell
- Pluripotent Stem Cell
- Cancer Stem Cell
- Adult Stem Cell
- Non-Stem Cell
- T Cells
- Natural Killer
- Others
- Stem Cell
- Gene Therapy
The report has provided a detailed breakup and analysis of the market based on the therapy type. This includes cell therapy {stem cell (pluripotent stem cell, cancer stem cell, and adult stem cell) non-stem cell (T cells, natural killer, and others)} and gene therapy.
Indication Insights:
- Cardiovascular Disease
- Oncology Disorder
- Genetic Disorder
- Infectious Disease
- Neurological Disorder
- Others
A detailed breakup and analysis of the market based on the indication have also been provided in the report. This includes cardiovascular disease, oncology disorder, genetic disorder, infectious disease, neurological disorder, and others.
Delivery Mode Insights:
- In-Vivo
- Ex-Vivo
A detailed breakup and analysis of the market based on the delivery mode have also been provided in the report. This includes in-vivo and ex-vivo.
End User Insights:
Access the comprehensive market breakdown Request Sample
- Hospitals
- Cancer Care Centers
- Pharmaceutical & Biotechnology Companies
- Others
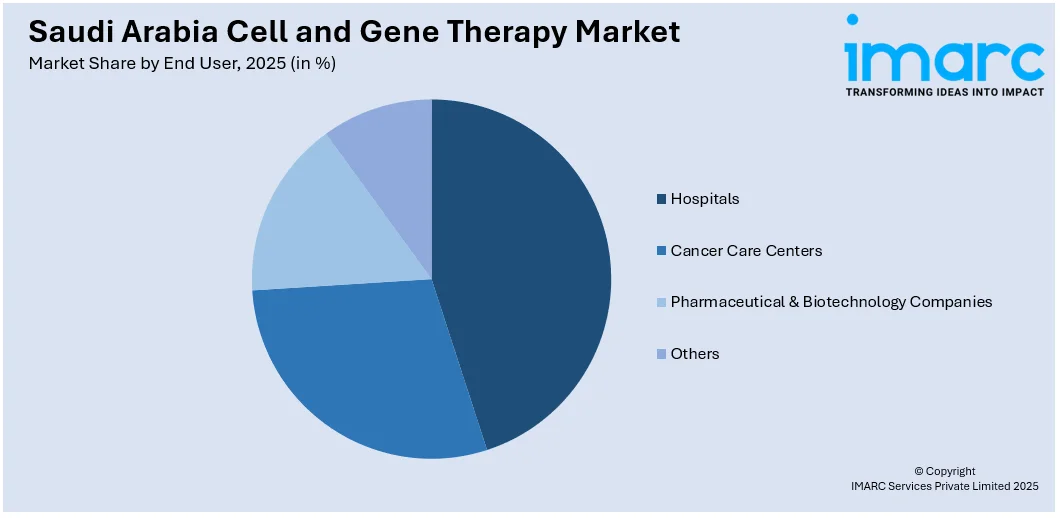
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes hospitals, cancer care centers, pharmaceutical & biotechnology companies, and others.
Regional Insights:
- Northern and Central Region
- Western Region
- Eastern Region
- Southern Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Northern and Central region, Western region, Eastern region, and Southern region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Saudi Arabia Cell and Gene Therapy Market News:
- January 2025: Charles River partnered with Lafana Holding to launch the Saudi Cell and Gene Therapy Accelerator Program. This initiative supported biotech ecosystem growth by offering CDMO services, investment aid, and advisory support, advancing Saudi Arabia’s capabilities and access in the gene therapy sector.
- December 2024: Vertex Pharmaceuticals signed an MoU with Saudi Arabia’s ministries to boost local gene therapy manufacturing. The USD 266 Million initiative supported R&D, production, and workforce training, advancing access to therapies like Casgevy and strengthening Saudi Arabia’s position in the cell and gene therapy sector.
Saudi Arabia Cell and Gene Therapy Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Therapy Types Covered |
|
| Indications Covered | Cardiovascular Disease, Oncology Disorder, Genetic Disorder, Infectious Disease, Neurological Disorder, Others |
| Delivery Modes Covered | In-Vivo, Ex-Vivo |
| End Users Covered | Hospitals, Cancer Care Centers, Pharmaceutical & Biotechnology Companies, Others |
| Regions Covered | Northern and Central Region, Western Region, Eastern Region, Southern Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Saudi Arabia cell and gene therapy market performed so far and how will it perform in the coming years?
- What is the breakup of the Saudi Arabia cell and gene therapy market on the basis of the therapy type?
- What is the breakup of the Saudi Arabia cell and gene therapy market on the basis of indication?
- What is the breakup of the Saudi Arabia cell and gene therapy market on the basis of delivery mode?
- What is the breakup of the Saudi Arabia cell and gene therapy market on the basis of end user?
- What is the breakup of the Saudi Arabia cell and gene therapy market on the basis of region?
- What are the various stages in the value chain of the Saudi Arabia cell and gene therapy market?
- What are the key driving factors and challenges in the Saudi Arabia cell and gene therapy market?
- What is the structure of the Saudi Arabia cell and gene therapy market and who are the key players?
- What is the degree of competition in the Saudi Arabia cell and gene therapy market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Saudi Arabia cell and gene therapy market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Saudi Arabia cell and gene therapy market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Saudi Arabia cell and gene therapy industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












