
Saudi Arabia Cell Therapy Market Size, Share, Trends and Forecast by Cell Type, Therapy Type, Therapeutic Area, End User, and Region, 2026-2034
Saudi Arabia Cell Therapy Market Overview:
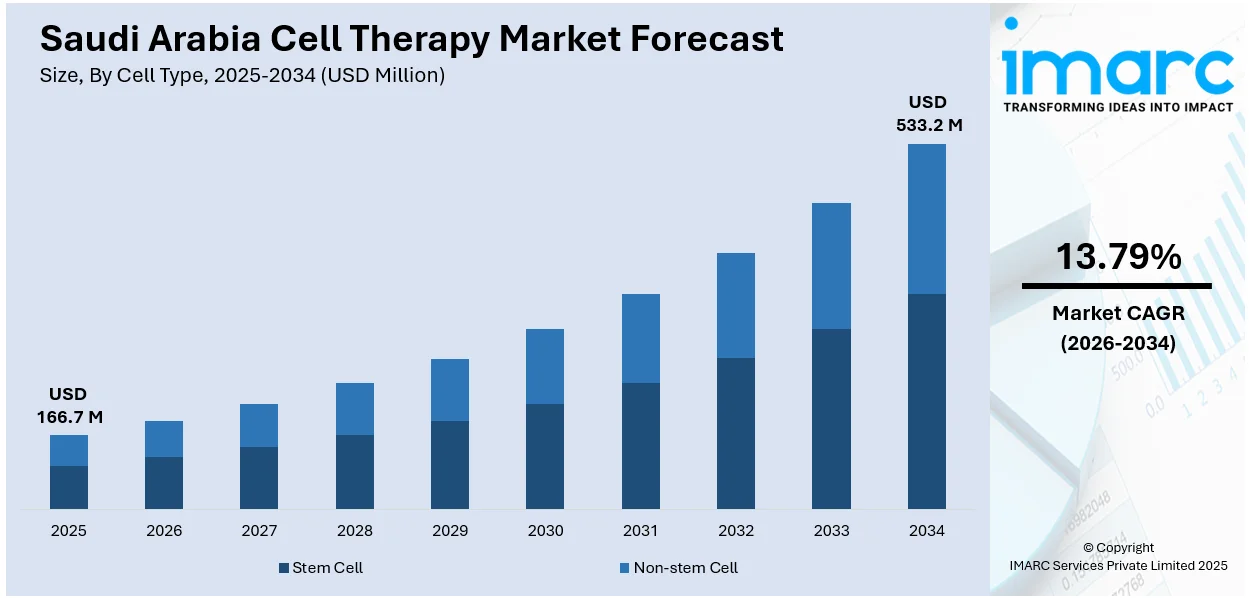
The Saudi Arabia cell therapy market size reached USD 166.7 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 533.2 Million by 2034, exhibiting a growth rate (CAGR) of 13.79% during 2026-2034. Government investments in healthcare infrastructure, establishment of research centers, development of clinical laboratories, and partnerships with global biopharmaceutical companies are key factors driving the market. Rising prevalence of chronic diseases, growing incidence of genetic disorders, and increasing demand for regenerative treatments are additional market drivers. Regulatory advancements and medical education improvements are some of the factors positively impacting the Saudi Arabia cell therapy market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 166.7 Million |
| Market Forecast in 2034 | USD 533.2 Million |
| Market Growth Rate 2026-2034 | 13.79% |
Saudi Arabia Cell Therapy Market Trends:
Increasing Prioritization of Advanced Healthcare Infrastructure
Saudi Arabia’s Vision 2030 initiative places significant emphasis on diversifying the economy and investing in sectors beyond oil, with healthcare representing a major focus area. Extensive government-led programs are leading to the development of state-of-the-art research centers, clinical laboratories, and biotechnology facilities. This strong institutional support is enabling greater access to cutting-edge treatments, including various cell therapy modalities, for conditions such as cancer, autoimmune diseases, and genetic disorders. Additionally, partnerships between public entities and global biopharmaceutical companies are introducing international best practices and technological advancements into the domestic market. Substantial investments in medical education and research are equipping a new generation of healthcare professionals with the necessary expertise to advance cell therapy applications. On January 9, 2024, Vertex Pharmaceuticals announced that the Saudi Food and Drug Authority (SFDA) granted marketing authorization for CASGEVY™ (exagamglogene autotemcel), a CRISPR/Cas9 gene-edited therapy for sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). This approval marks the first use of the SFDA's Breakthrough Medicines Program and Vertex's first regulatory approval in Saudi Arabia. Notably, these developments are directly contributing to Saudi Arabia cell therapy market growth, as the enhanced infrastructure attracts both domestic and foreign stakeholders interested in the cell therapy sector. Furthermore, strategic collaborations with academic institutions are accelerating innovation, ensuring that the local ecosystem remains competitive on the global stage. The clear government commitment towards healthcare modernization is creating fertile ground for the sustained expansion of cell therapy solutions across Saudi Arabia.
To get more information on this market Request Sample
Rising Prevalence of Chronic and Rare Diseases Among the Population
Lifestyle-related conditions such as diabetes, cardiovascular disorders, and obesity are widespread, necessitating more personalized and regenerative treatment options. In addition, genetic disorders, particularly those prevalent among specific regional demographics, are prompting a growing need for innovative therapeutic approaches. Patients and healthcare providers are seeking alternatives to traditional pharmaceutical treatments, looking toward regenerative medicine to offer more durable and targeted outcomes. The aging population is another important aspect contributing to the demand for advanced therapies that can address degenerative conditions more effectively. On April 23, 2025, CEL-SCI Corporation announced its intention to file for regulatory approval of its Multikine cancer immunotherapy in Saudi Arabia, aiming for Conditional Approval with Breakthrough Therapy designation based on Phase 3 data. The company is exploring partnerships with local Saudi firms to support commercialization, clinical trials, and establish a manufacturing facility for Multikine to serve the Middle East and North Africa (MENA) region. Within this landscape, the market trends demonstrate a shift in both public and private healthcare providers’ procurement strategies, focusing on next-generation therapies. Advances in cell-based immunotherapies and stem cell research are further enhancing treatment possibilities, supporting a gradual transformation in clinical care models. Moreover, regulatory bodies in the Kingdom are working to create frameworks that facilitate safe and efficient clinical adoption of cell therapy products, reinforcing confidence among investors and practitioners. As a result, these medical and regulatory dynamics are redefining therapeutic protocols and stimulating broader acceptance of cell therapy in the national healthcare agenda.
Saudi Arabia Cell Therapy Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2026-2034. Our report has categorized the market based on cell type, therapy type, therapeutic area, and end user.
Cell Type Insights:
- Stem Cell
- Bone Marrow
- Blood
- Umbilical Cord-Derived
- Adipose-Derived Stem Cell
- Others
- Non-stem Cell
The report has provided a detailed breakup and analysis of the market based on the cell type. This includes stem cell (bone marrow, blood, umbilical cord-derived, adipose-derived stem cell, and others) and non-stem cell.
Therapy Type Insights:
- Autologous
- Allogeneic
The report has provided a detailed breakup and analysis of the market based on the therapy type. This includes autologous and allogeneic.
Therapeutic Area Insights:
- Malignancies
- Musculoskeletal Disorders
- Autoimmune Disorders
- Dermatology
- Others
The report has provided a detailed breakup and analysis of the market based on the therapeutic area. This includes malignancies, musculoskeletal disorders, autoimmune disorders, dermatology, and others.
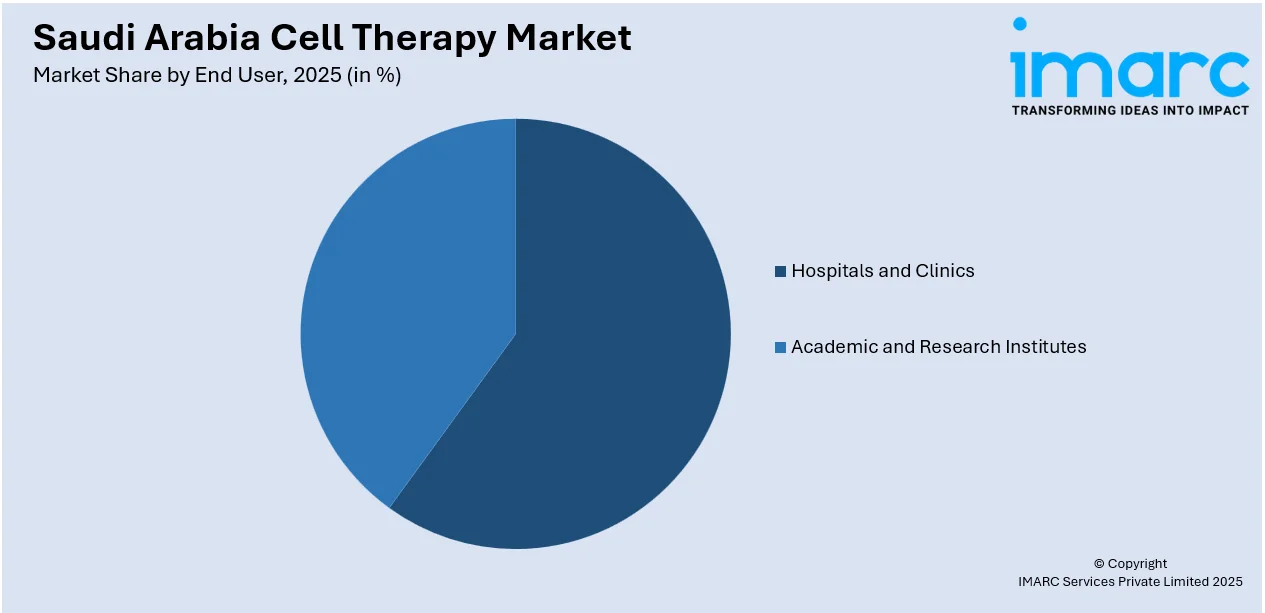
End User Insights:
Access the comprehensive market breakdown Request Sample
- Hospitals and Clinics
- Academic and Research Institutes
The report has provided a detailed breakup and analysis of the market based on the end user. This includes hospitals and clinics and academic and research institutes.
Regional Insights:
- Northern and Central Region
- Western Region
- Eastern Region
- Southern Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Northern and Central Region, Western Region, Eastern Region, and Southern Region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Saudi Arabia Cell Therapy Market News:
- On April 27, 2025, Fakeeh Care signed a memorandum of understanding (MoU) with Shanghai Fosun Pharmaceutical to advance cell and gene therapies, including CAR-T, in Saudi Arabia. The partnership aims to enhance the Kingdom's healthcare landscape by introducing AI-powered digital pathology and remote diagnostic services, aligning with Saudi Vision 2030.
Saudi Arabia Cell Therapy Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Cell Types Covered |
|
| Therapy Types Covered | Autologous, Allogeneic |
| Therapeutic Areas Covered | Malignancies, Musculoskeletal Disorders, Autoimmune Disorders, Dermatology, Others |
| End Users Covered | Hospitals and Clinics, Academic and Research Institutes |
| Regions Covered | Northern and Central Region, Western Region, Eastern Region, Southern Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Saudi Arabia cell therapy market performed so far and how will it perform in the coming years?
- What is the breakup of the Saudi Arabia cell therapy market on the basis of cell type?
- What is the breakup of the Saudi Arabia cell therapy market on the basis of therapy type?
- What is the breakup of the Saudi Arabia cell therapy market on the basis of therapeutic area?
- What is the breakup of the Saudi Arabia cell therapy market on the basis of end user?
- What is the breakup of the Saudi Arabia cell therapy market on the basis of region?
- What are the various stages in the value chain of the Saudi Arabia cell therapy market?
- What are the key driving factors and challenges in the Saudi Arabia cell therapy market?
- What is the structure of the Saudi Arabia cell therapy market and who are the key players?
- What is the degree of competition in the Saudi Arabia cell therapy market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Saudi Arabia cell therapy market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Saudi Arabia cell therapy market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Saudi Arabia cell therapy industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












