
Saudi Arabia DNA Testing Market Size, Share, Trends and Forecast by Product Type, Technology, Application, and Region, 2026-2034
Saudi Arabia DNA Testing Market Overview:
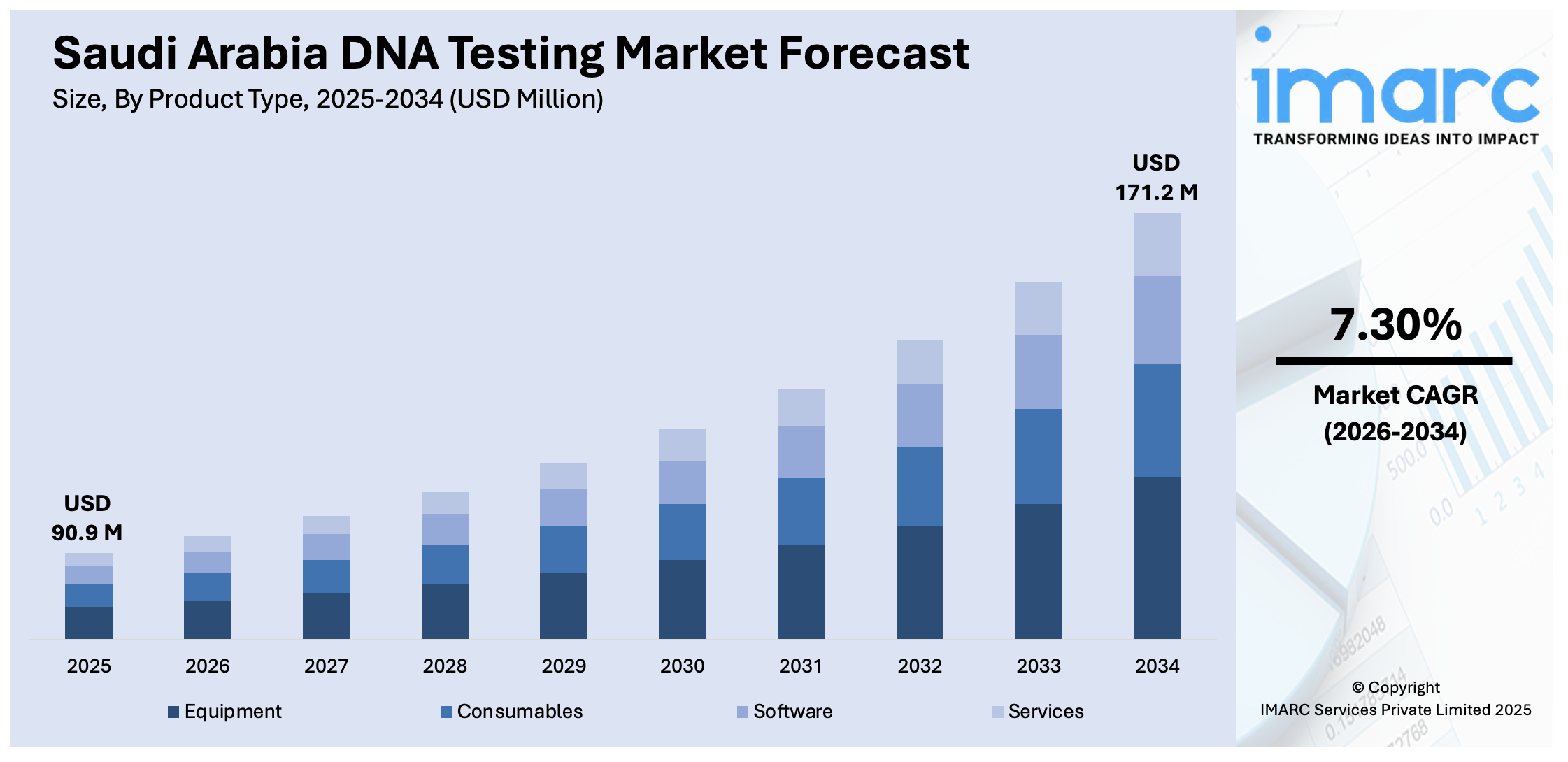
The Saudi Arabia DNA testing market size reached USD 90.9 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 171.2 Million by 2034, exhibiting a growth rate (CAGR) of 7.30% during 2026-2034. The market is expanding due to factors such as increasing demand for personalized medicine, government support under Vision 2030, and rising awareness of preventive healthcare. Technological advancements in genomic research and expanding direct-to-consumer (DTC) genetic testing services further contribute to market growth. These factors collectively drive the Saudi Arabia DNA testing market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 90.9 Million |
| Market Forecast in 2034 | USD 171.2 Million |
| Market Growth Rate 2026-2034 | 7.30% |
Saudi Arabia DNA Testing Market Trends:
Integration of Artificial Intelligence in Genetic Testing
Artificial Intelligence (AI) is transforming the Saudi Arabia DNA testing industry by improving the interpretation of sophisticated genetic information. AI software makes it easier to spot patterns in the genes, allowing for more precise diagnostics and customized treatment programs. This support enables early genetic disorder detection and maximizes therapeutic plans, adding to the growth of the market. As the technology of AI keeps evolving, its application in genomic studies and diagnostics is likely to increase, further driving the Saudi Arabia DNA testing market expansion. For instance, in July 2023, Germany’s Centogene and Saudi Arabia’s Lifera (a PIF-owned company) announced a $30 million joint venture to expand access to advanced multiomic testing in Saudi Arabia and the GCC. The partnership supports Saudi Vision 2030, aiming to enhance local genomic capabilities. Leveraging Centogene’s vast Biodatabank and Lifera’s regional presence, the venture will establish a cutting-edge lab and bioinformatics infrastructure. It will drive national screening programs, improve diagnostics, and bolster the Saudi biotech sector and public health resilience.
To get more information on this market Request Sample
Government Initiatives and Vision 2030 Support
The Saudi Arabian government's Vision 2030 initiative plays a pivotal role in advancing the DNA testing market. Through investments in healthcare infrastructure and support for genomics research, the government fosters an environment conducive to the growth of genetic testing services. Programs like the Saudi Genome Program (SGP) aims to revolutionize healthcare through personalized medicine by creating a comprehensive genetic database of the Saudi population. It focuses on reducing genetic diseases, improving diagnosis and treatments, and building national genomic capabilities. With over 63,000 samples processed and 140+ researchers involved, SGP is advancing toward becoming a global leader in genomics. The program is key to transforming healthcare in Saudi Arabia and positioning it as a hub for genomic innovation. These initiatives enhance accessibility to genetic testing, thereby accelerating the Saudi Arabia DNA testing market growth.
Saudi Arabia DNA Testing Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country/regional levels for 2026-2034. Our report has categorized the market based on product type, technology, and application.
Product Type Insights:
- Equipment
- DNA Analyzers
- PCR (Polymerase Chain Reaction) Machines
- Sequencers
- Electrophoresis Systems
- Others
- Consumables
- Reagents and Kits
- PCR Consumables
- Primers
- Probes
- DNA Extraction Kits
- Sample Collection Devices
- Others
- Software
- Data Analysis Software
- Laboratory Information Management System (LIMS)
- Genetic Analysis Software
- Bioinformatics Software
- Others
- Services
- Testing Services
- Genetic Testing
- Forensic Testing
- Others
- Data Interpretation Services
- Consultation Services
- Genetic Counseling
- Laboratory Services
- Sample Processing
- DNA Sequencing Services
- Others
- Testing Services
The report has provided a detailed breakup and analysis of the market based on the product type. This includes equipment (DNA analyzers, PCR (polymerase chain reaction) machines, sequencers, electrophoresis systems, and others), consumables (reagents and kits and PCR consumables (primers, probes, DNA extraction kits, sample collection devices, and others)), software (data analysis software, laboratory information management system (LIMS), genetic analysis software, bioinformatics software, and others), and services (testing services (genetic testing, forensic testing, and others), data interpretation services, consultation services (genetic counseling), laboratory services (sample processing and DNA sequencing services), and others)).
Technology Insights:
- Polymerase Chain Reaction (PCR) Based
- In-Situ Hybridization
- Microarray
- Next-Generation Sequencing (NGS) DNA Diagnosis
A detailed breakup and analysis of the market based on the technology have also been provided in the report. This includes polymerase chain reaction (PCR) based, in-situ hybridization, microarray, and next-generation sequencing (NGS) DNA diagnosis.
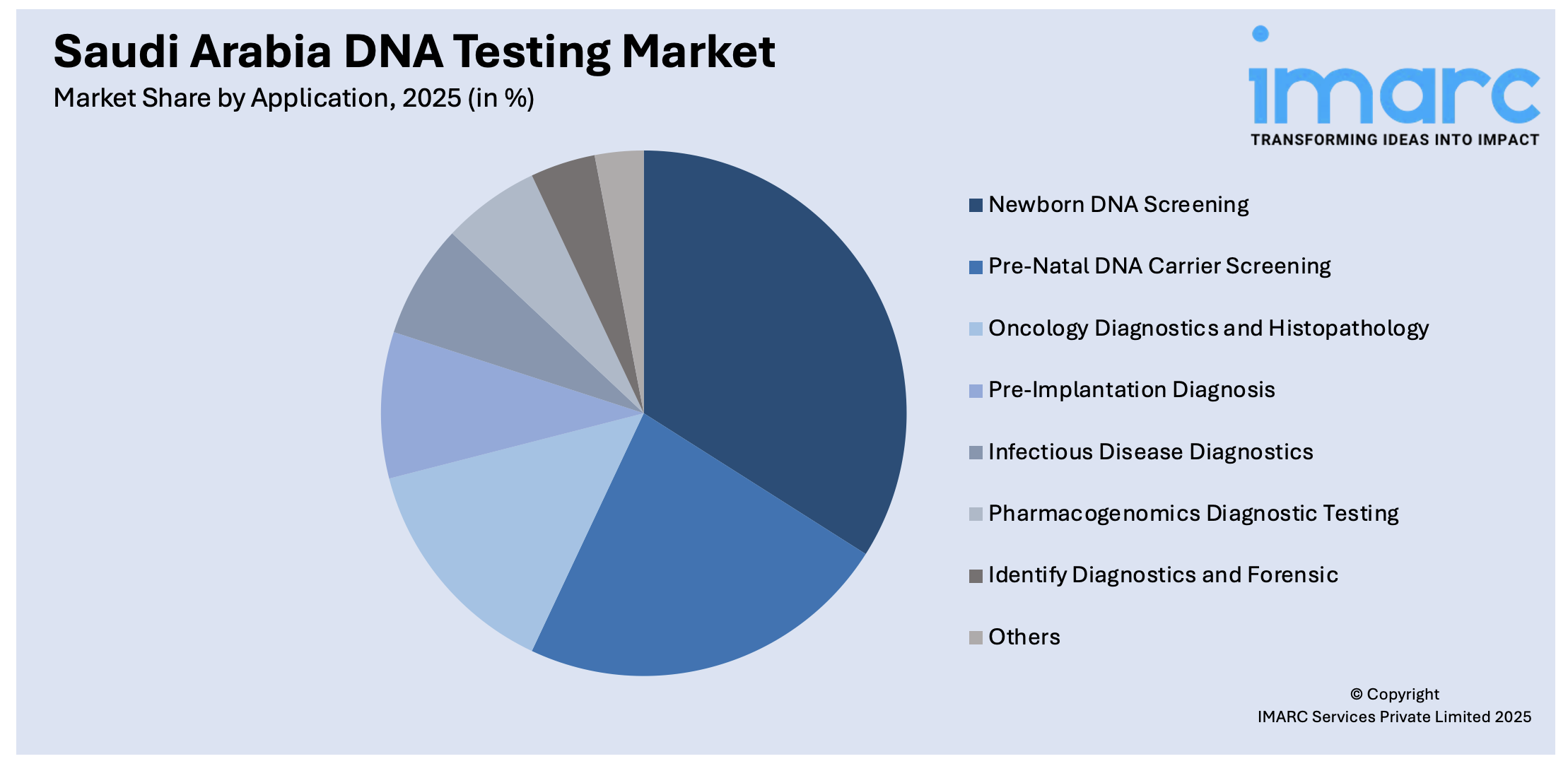
Application Insights:
Access the comprehensive market breakdown Request Sample
- Newborn DNA Screening
- Pre-Natal DNA Carrier Screening
- Oncology Diagnostics and Histopathology
- Pre-Implantation Diagnosis
- Infectious Disease Diagnostics
- Pharmacogenomics Diagnostic Testing
- Identify Diagnostics and Forensic
- Others
A detailed breakup and analysis of the market based on the application have also been provided in the report. This includes newborn DNA screening, pre-natal DNA carrier screening, oncology diagnostics and histopathology, pre-implantation diagnosis, infectious disease diagnostics, pharmacogenomics diagnostic testing, identity diagnostics and forensic, and others.
Regional Insights:
- Northern and Central Region
- Western Region
- Eastern Region
- Southern Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Northern and Central Region, Western Region, Eastern Region, and Southern Region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Saudi Arabia DNA Testing Market News:
- In March 2025, BGI Genomics' joint venture, Genalive, secured a 950 million RMB contract from Saudi Arabia’s National Unified Procurement Company (NUPCO) for outsourced genetic testing services over three years. This is NUPCO’s largest tender to date, covering 930,000 tests across 83 public hospitals. Services include WGS, WES, NIPT, and hereditary cancer screening. The partnership aims to enhance Saudi Arabia’s precision medicine capabilities, integrating China’s genomics technology with local healthcare infrastructure to strengthen the country's public health and diagnostic services.
- In February 2025, OncoDNA, Bayer Saudi Arabia, and Tamkin Al-Seha Medical partnered to expand access to NTRK biomarker testing in Saudi Arabia. The initiative aims to screen 1,000 patients with advanced tumors for NTRK gene fusions using OncoDNA’s next-generation sequencing technologies. The collaboration aligns with Saudi Arabia’s healthcare innovation goals and provides a model for localized adoption of advanced cancer diagnostics and targeted treatment approaches.
Saudi Arabia DNA Testing Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Product Types Covered |
|
| Technologies Covered | Polymerase Chain Reaction (PCR) Based, In-Situ Hybridization, Microarray, Next-Generation Sequencing (NGS) DNA Diagnosis |
| Applications Covered | Newborn DNA Screening, Pre-Natal DNA Carrier Screening, Oncology Diagnostics and Histopathology, Pre-Implantation Diagnosis, Infectious Disease Diagnostics, Pharmacogenomics Diagnostic Testing, Identity Diagnostics and Forensic, Others |
| Regions Covered | Northern and Central Region, Western Region, Eastern Region, Southern Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Saudi Arabia DNA testing market performed so far and how will it perform in the coming years?
- What is the breakup of the Saudi Arabia DNA testing market on the basis of product type?
- What is the breakup of the Saudi Arabia DNA testing market on the basis of technology?
- What is the breakup of the Saudi Arabia DNA testing market on the basis of application?
- What is the breakup of the Saudi Arabia DNA testing market on the basis of region?
- What are the various stages in the value chain of the Saudi Arabia DNA testing market?
- What are the key driving factors and challenges in the Saudi Arabia DNA testing market?
- What is the structure of the Saudi Arabia DNA testing market and who are the key players?
- What is the degree of competition in the Saudi Arabia DNA testing market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Saudi Arabia DNA testing market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Saudi Arabia DNA testing market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Saudi Arabia DNA testing industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












