
Saudi Arabia Sterilization Equipment Market Size, Share, Trends and Forecast by Product, End User, and Region, 2026-2034
Saudi Arabia Sterilization Equipment Market Overview:
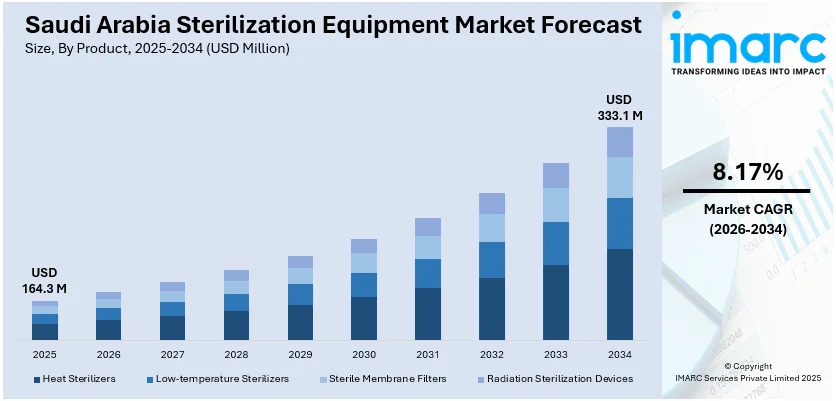
The Saudi Arabia sterilization equipment market size reached USD 164.3 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 333.1 Million by 2034, exhibiting a growth rate (CAGR) of 8.17% during 2026-2034. The market is driven by rising healthcare infrastructure investments, increasing surgical procedures, strict infection control protocols, and the expansion of pharmaceutical and biotech manufacturing. Government initiatives supporting medical device localization and the demand for safe, reusable instruments are further encouraging sterilization technology adoption.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 164.3 Million |
| Market Forecast in 2034 | USD 333.1 Million |
| Market Growth Rate 2026-2034 | 8.17% |
Saudi Arabia Sterilization Equipment Market Trends:
Growing Role of Sterilization in Local Pharmaceutical Manufacturing
Saudi Arabia’s push toward pharmaceutical self-sufficiency under Vision 2030 is fueling the adoption of sterilization equipment in drug manufacturing. As domestic production of injectables, biologics, and sterile dosage forms expands, regulatory compliance with GMP (Good Manufacturing Practices) is becoming a critical priority. This requires reliable sterilization solutions such as depyrogenation ovens, autoclaves, and sterile membrane filters to ensure product safety and shelf stability. Additionally, multinational pharmaceutical firms establishing regional manufacturing hubs in Saudi Arabia are adopting global hygiene standards, further boosting demand. This trend is reinforced by partnerships between local players and international equipment suppliers, aiming to strengthen the country’s position as a regional pharmaceutical production center with robust sterilization protocols in place. For instance, Syntegon, through its subsidiary Schoeller-Bleckmann Medizintechnik (SBM), offers advanced pharmaceutical sterilization solutions using vacuum-steam, steam-air, and hot water shower technologies. These modular autoclaves are designed for sterilizing porous materials, liquids, and pharmaceutical containers with flexibility and energy efficiency. Key product lines, ADV, SDR, and SWS, serve various container types and include features like automatic in-line filter sterilization and FDA-compliant components. With over 1,500 units installed globally, Syntegon emphasizes hygienic design, process reliability, and customizable configurations to meet modern pharmaceutical sterilization demands.
To get more information on this market Request Sample
Integration of Advanced Sterilization Equipment in Hospital Infrastructure
The Saudi healthcare system is undergoing modernization with new hospitals, clinics, and surgical centers incorporating advanced sterilization units. With the growing number of complex procedures and patient safety regulations, facilities are increasingly investing in steam autoclaves, low-temperature hydrogen peroxide sterilizers, and ethylene oxide systems. These technologies are being installed in both centralized sterile supply departments and decentralized operating suites to ensure uninterrupted workflow. Emphasis is also placed on energy-efficient, automated systems that meet international infection control standards. The rise of medical tourism in cities like Riyadh and Jeddah further increases expectations for top-tier hygiene practices, making sterilization equipment an essential component of new hospital projects and expansions across the Kingdom. For instance, in February 2024, Arabian Medical Products Manufacturing Company (ENAYAH) achieved Medical Device Regulation (MDR) certification from SGS in Saudi Arabia. ENAYAH produces single-use healthcare products. The MDR certification signifies ENAYAH's commitment to high product quality and patient safety, strengthening its global market presence, enhancing customer trust, and improving operational efficiency. SGS provides certification and training solutions for the medical device industry in Saudi Arabia.
Saudi Arabia Sterilization Equipment Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country/regional levels for 2026-2034. Our report has categorized the market based on product and end user.
Product Insights:
- Heat Sterilizers
- Depyrogenation Oven
- Steam Autoclaves
- Low-temperature Sterilizers
- Ethylene Oxide Sterilizers
- Hydrogen Peroxide Sterilizers
- Others
- Sterile Membrane Filters
- Radiation Sterilization Devices
- Electron Beams
- Gamma Rays
- Others
The report has provided a detailed breakup and analysis of the market based on the product. This includes heat sterilizers (depyrogenation ovens and steam autoclaves), low-temperature sterilizers (ethylene oxide sterilizers, hydrogen peroxide sterilizers, and others), sterile membrane filters, and radiation sterilization devices (electron beams, gamma rays, and others).
End User Insights:
Access the comprehensive market breakdown Request Sample
- Hospitals and Clinics
- Medical Device Companies
- Pharmaceutical Companies
- Food and Beverages Industry
- Others
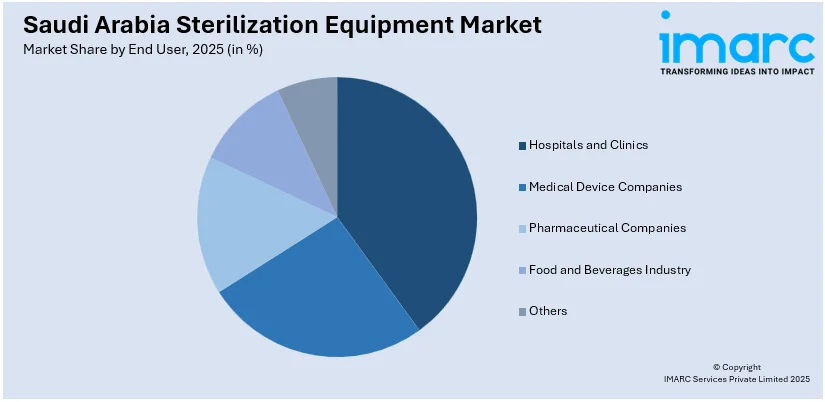
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes hospitals and clinics, medical device companies, pharmaceutical companies, food and beverages industry, and others.
Regional Insights:
- Northern and Central Region
- Western Region
- Eastern Region
- Southern Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Northern and Central Region, Western Region, Eastern Region, and Southern Region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Saudi Arabia Sterilization Equipment Market News:
- In March 2025, COLTENE Group launched new dental solutions at IDS 2025, including innovations in infection control, endodontics, and restorative dentistry. These solutions aim to enhance clinical outcomes, improve workflows, and ensure instrument sterilization. New products include HySolate Liquid Dam, Kenda ShapeGuard, MicroMega One Glider & RECI Glider, Roeko Gelatamp forte & white, and BRILLIANT Lumina. COLTENE focuses on providing comprehensive, high-quality solutions for dental professionals.
Saudi Arabia Sterilization Equipment Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered |
|
| End Users Covered | Hospitals and Clinics, Medical Device Companies, Pharmaceutical Companies, Food and Beverages Industry, Others |
| Regions Covered | Northern and Central Region, Western Region, Eastern Region, Southern Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Saudi Arabia sterilization equipment market performed so far and how will it perform in the coming years?
- What is the breakup of the Saudi Arabia sterilization equipment market on the basis of product?
- What is the breakup of the Saudi Arabia sterilization equipment market on the basis of end user?
- What is the breakup of the Saudi Arabia sterilization equipment market on the basis of region?
- What are the various stages in the value chain of the Saudi Arabia sterilization equipment market?
- What are the key driving factors and challenges in the Saudi Arabia sterilization equipment market?
- What is the structure of the Saudi Arabia sterilization equipment market and who are the key players?
- What is the degree of competition in the Saudi Arabia sterilization equipment market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Saudi Arabia sterilization equipment market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Saudi Arabia sterilization equipment market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Saudi Arabia sterilization equipment industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












