
South East Asia Biopreservation Market Size, Share, Trends and Forecast by Product Type, Application, and Country, 2026-2034
South East Asia Biopreservation Market Summary:
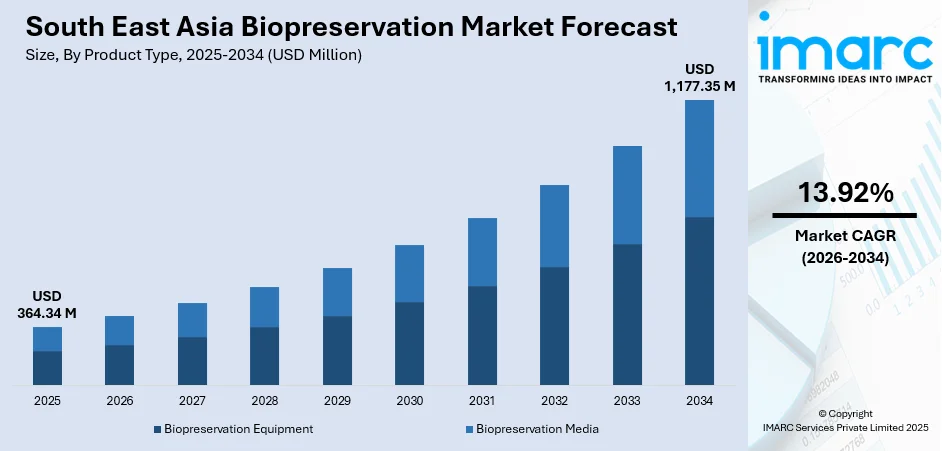
The South East Asia biopreservation market size was valued at USD 364.34 Million in 2025 and is projected to reach USD 1,177.35 Million by 2034, growing at a compound annual growth rate of 13.92% from 2026-2034.
The market is poised for steady growth driven by rising demand for long term biological sample storage, expanding clinical research activity, and increased investments in biobanking infrastructure. Adoption of advanced cryopreservation and cold chain technologies is improving sample integrity while regulatory focus and public private collaborations support market maturation and commercialization opportunities across the region over the forecast period.
Key Takeaways and Insights:
- By Product Type: Biopreservation equipment dominates the market with a share of 58% in 2025, driven by substantial capital investments in ultra-low temperature freezers, controlled-rate cooling systems, and automated cryogenic storage infrastructure across regional biobanks and research facilities.
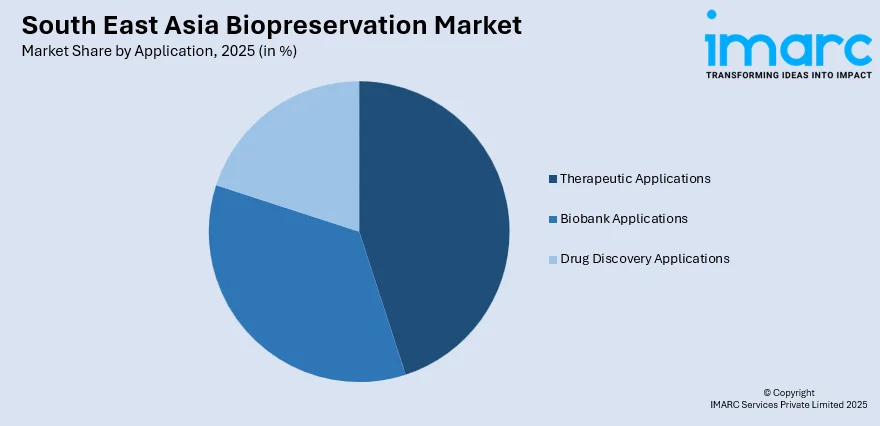
- By Application: Therapeutic applications lead the market with a share of 45% in 2025, propelled by the expanding cell and gene therapy sector, growing stem cell research initiatives, and increasing adoption of regenerative medicine treatments across major healthcare facilities in the region.
- Key Players: The South East Asia biopreservation market exhibits a moderately competitive landscape with established global manufacturers collaborating with regional distributors. International players are strengthening their presence through strategic partnerships, technology licensing agreements, and localized manufacturing initiatives to capture growing demand across therapeutic and research applications.
To get more information on this market Request Sample
The South East Asia biopreservation market is experiencing sustained expansion supported by rising demand for high quality biological material storage across pharmaceutical, biotechnology, and diagnostics applications. In October 2025, NSG Bio launched NSG BioSuites, its largest facility in Singapore, located in the Geneo development. Designed as an affordable, BSL-2 certified space with shared amenities, it strengthens access to purpose built lab infrastructure and supports diverse biotech workflows. Growing research funding, increased clinical trial activity, and the shift toward personalized therapies are further accelerating adoption of controlled temperature preservation systems. Countries across the region are also enhancing biobanking networks to advance regenerative medicine, cell therapy development, and translational research, increasing the need for reliable storage solutions. Technological improvements in cryogenic equipment, preservation media, and cold chain logistics continue to boost sample integrity and operational efficiency. Combined with a strengthening healthcare ecosystem, evolving regulatory frameworks, and deeper industry–research collaboration, biopreservation is becoming a critical foundation for regional scientific and commercial progress.
South East Asia Biopreservation Market Trends:
Growing Focus on Regenerative Medicine
The South East Asia biopreservation market is benefiting from rising interest in regenerative medicine, supported by expanding research in stem cells, tissue engineering, and cell based therapies. In July 2025, Life Biosciences partnered with SingHealth Duke-NUS Regenerative Medicine Institute in Singapore to enhance its cellular rejuvenation therapies targeting age-related diseases. The collaboration aims to leverage REMEDIS's expertise and Life Bio’s Partial Epigenetic Reprogramming Platform, advancing innovative treatments for healthier aging and improving patient care outcomes worldwide. This shift is increasing the need for reliable preservation systems that maintain cell functionality and viability over extended periods. As therapeutic pipelines diversify, institutions are prioritizing high quality cryopreservation, controlled temperature solutions, and standardized storage protocols to support clinical readiness and future commercialization opportunities.
Expansion of Regional Biobanking Networks
Biobanking capacity across South East Asia is expanding as research institutes, healthcare systems, and private organizations strengthen their infrastructure to support advanced scientific programs. This growth is elevating demand for sophisticated temperature-controlled storage, optimized preservation media, and well managed sample handling workflows. In October 2025, Haier Biomedical installed five advanced Blood Banking Centrifuges at Hospital R in Ho Chi Minh City, Vietnam, enhancing local healthcare capabilities. A comprehensive training program preceded the launch, ensuring staff proficiency. The LX-75L2400R centrifuge offers superior performance in blood separation, significantly improving processing efficiency and safety in biomedical applications. The expansion of biobanks is also enabling broader collaboration in precision medicine, accelerating research outputs, and reinforcing the role of biopreservation as a foundational component of regional biomedical development.
Advancements in Cryogenic and Cold Chain Technologies
Technological progress in cryogenic storage and cold chain management is reshaping operational standards within the South East Asia biopreservation market. New generation freezers, automated sample storage systems, and improved monitoring solutions are helping preserve sample integrity more reliably. Enhanced cold chain logistics are also reducing risks during transport and long-term storage. In November 2024, Envirotainer launched a new CryoSure® station in Singapore, enhancing its ultra-cold shipping network for pharmaceuticals at -70°C across Asia and the Pacific. This facility, strategically located near a major cargo airport, improves logistics efficiency and sustainability while supporting the growing demand for cell and gene therapies. These innovations support greater scalability, reduce operational inefficiencies, and enable research organizations to maintain consistent preservation quality across diverse workflows.
Market Outlook 2026-2034:
The South East Asia biopreservation market outlook remains positive, supported by expanding research activity, rising adoption of advanced therapies, and strengthening biobanking infrastructure across the region. Growing investment in life sciences, combined with advancements in cryogenic technologies and cold chain systems, will continue to enhance storage reliability and operational efficiency. As clinical trials, personalized medicine, and regenerative therapies progress, the market is expected to experience steady growth, driven by increasing demand for high quality long term biological sample preservation solutions. The market generated a revenue of USD 364.34 Million in 2025 and is projected to reach a revenue of USD 1,177.35 Million by 2034, growing at a compound annual growth rate of 13.92% from 2026-2034.
South East Asia Biopreservation Market Report Segmentation:
| Segment Category | Leading Segment | Market Share |
|---|---|---|
| Product Type | Biopreservation Equipment | 58% |
| Application | Therapeutic Applications | 45% |
Product Type Insights:
- Biopreservation Equipment
- Biopreservation Media
The biopreservation equipment dominates with a market share of 58% of the total South East Asia biopreservation market in 2025.
The biopreservation equipment segment benefits from rising adoption of advanced cryogenic freezers, automated storage platforms, and temperature monitoring systems that support long term sample integrity. Growing research intensity across biotechnology and pharmaceutical sectors is further accelerating the need for dependable, scalable, and compliant equipment capable of managing diverse biological materials under controlled conditions.
Demand for biopreservation equipment continues to strengthen as institutions upgrade infrastructure to support expanding biobanking activity, regenerative medicine programs, and personalized therapy development. In September 2025, Innovate Biotech Co., Ltd. becomes Thailand’s first private biobank to achieve ISO 20387:2018 certification, enhancing its credibility in biological resource management. The certification supports a new biobanking business model for human samples, positioning Thailand as a future Medical Hub of Asia. Increasing preference for automation, improved energy efficiency, and enhanced safety features is shaping investment decisions across laboratories and research centers. These advancements are elevating operational reliability and helping organizations streamline sample handling practices, making equipment the primary revenue generating category within the regional biopreservation landscape.
Application Insights:
Access the Comprehensive Market Breakdown Request Sample
- Therapeutic Applications
- Biobank Applications
- Drug Discovery Applications
The therapeutic applications lead with a share of 45% of the total South East Asia biopreservation market in 2025.
Therapeutic applications lead the South East Asia biopreservation, driven by the rapid growth of cell based therapies, gene therapies, and regenerative medicine programs. In September 2025, eXmoor Pharma and Siam Bioscience partnered to establish a leading center for cell and gene therapy in Southeast Asia. This collaboration aims to enhance local innovation and attract global therapies, improving patient access to advanced medicines while supporting the growth of sustainable healthcare systems in the region. These applications require high quality preservation systems to maintain viability, potency, and safety of therapeutic grade materials. As healthcare providers and research organizations expand their therapeutic pipelines, demand for robust cryogenic storage and standardized preservation protocols continues to rise across the region.
The dominance of therapeutic applications is further reinforced by increasing clinical trial activity and growing emphasis on personalized treatment approaches. Long term storage of stem cells, immune cells, and other therapeutic candidates requires consistent temperature control and validated workflows, prompting greater reliance on advanced preservation solutions. This trend is strengthening the segment’s position and supporting wider integration of biopreservation technologies within evolving therapeutic development strategies.
Country Insights:
- Indonesia
- Thailand
- Singapore
- Philippines
- Vietnam
- Malaysia
- Others
Indonesia is witnessing steady adoption of biopreservation solutions supported by expanding biotechnology activity, growing clinical research programs, and rising investment in laboratory infrastructure. Strengthening healthcare capabilities and increasing interest in regenerative therapies are contributing to the country’s growing reliance on controlled temperature storage systems.
Thailand’s biopreservation market is growing as research institutions and healthcare providers expand capabilities in cell therapy, biobanking, and pharmaceutical development. Demand for reliable storage solutions is rising alongside advancements in clinical trials, regenerative medicine, and academic research, supported by improving laboratory infrastructure.
Singapore continues to advance its position as a regional biotechnology hub, driving strong demand for high quality biopreservation systems. Expanding research facilities, supportive government initiatives, and increasing investment in advanced therapies are reinforcing the country’s adoption of modern storage and preservation technologies.
The Philippines is experiencing gradual growth in biopreservation driven by increasing healthcare modernization, expanding diagnostics capabilities, and rising participation in biomedical research. Strengthening laboratory networks and growing interest in cell based studies are supporting the need for dependable long term storage solutions.
Vietnam’s biopreservation market is supported by expanding pharmaceutical research, improving clinical laboratory capacity, and rising focus on biotechnology development. Growing engagement in translational research and increasing interest in personalized healthcare are contributing to the demand for reliable preservation infrastructure.
Malaysia is witnessing increasing adoption of biopreservation technologies as research universities, healthcare systems, and private biotechnology firms expand capabilities. Strengthening biobanking initiatives, growing investments in life sciences, and rising focus on advanced therapeutic research are shaping the country’s need for modern storage systems.
Market Dynamics:
Growth Drivers:
Why is the South East Asia Biopreservation Market Growing?
Rising Investment in Life Sciences Research
Growing investment in biotechnology, pharmaceuticals, and academic research is significantly strengthening the demand for reliable biopreservation solutions across South East Asia. As institutions expand research programs in genomics, immunology, and therapeutic development, the need for controlled temperature storage and long term sample protection continues to increase. In February 2025, Singapore officially launched the MedTech Catapult with a S$38 million investment to enhance life science innovation. This initiative supports medtech innovators in overcoming challenges in product development, regulatory approval, and manufacturing, ultimately bringing advanced medical devices to market and fostering local manufacturing partnerships. This investment is encouraging laboratories to adopt advanced preservation systems that ensure sample integrity, improve workflow efficiency, and support a broader range of scientific activities across the region.
Expansion of Clinical Trials and Personalized Medicine
The rise in clinical trial activity and the growing emphasis on personalized therapies are elevating the importance of secure biological material preservation. Researchers and healthcare providers require dependable storage systems to maintain the quality of patient derived samples, biomarkers, and therapeutic candidates over extended periods. In June 2025, Standard BioTools' SomaScan 11K Assay was selected by Singapore's PRECISE-SG100K to analyze 100,000 plasma samples, making it a key player in one of the world's largest population health studies. This collaboration aims to enhance precision medicine through extensive data collection and insights from Singapore's diverse population. This shift is advancing demand for standardized, high quality preservation processes that support trial reliability, regulatory compliance, and the development of individualized treatment strategies within the regional healthcare ecosystem.
Increasing Adoption of Cell and Gene Therapies
The expanding focus on cell and gene therapies is driving stronger reliance on specialized biopreservation systems that maintain therapeutic grade viability. As organizations work on developing next generation treatments, they require advanced cryogenic storage, optimized preservation media, and precise temperature-controlled workflows. This need is propelling the use of sophisticated preservation platforms that safeguard cell functionality and support the scale up of therapy development, positioning biopreservation as a critical enabler of emerging medical innovations in the region. The Singapore Cell and Gene Therapy Pan-Asia Summit (SCGT 2025) showcased advancements in therapeutic innovations from July 16-18, 2025, at Biopolis. Featuring leaders in the field, the event highlighted initiatives enhancing CAR-T manufacturing, engineered extracellular vesicles, and RNA-based therapies, underscoring Asia's collaborative push for accessible and affordable CGT solutions.
Market Restraints:
What Challenges the South East Asia Biopreservation Market is Facing?
High Cost of Advanced Preservation Equipment
The adoption of modern biopreservation systems is often constrained by high upfront costs associated with cryogenic freezers, automated storage solutions, and specialized preservation media. These technologies require substantial investment, making it difficult for smaller laboratories, emerging research centers, and budget limited institutions to upgrade their storage infrastructure. Ongoing maintenance expenses further add to financial pressure, limiting widespread implementation across the region and slowing modernization efforts in resource constrained settings.
Limited Technical Expertise and Skilled Workforce
Effective biopreservation requires specialized knowledge in handling cryogenic systems, managing temperature-controlled workflows, and implementing standardized preservation protocols. Many institutions face shortages of trained personnel capable of operating advanced equipment and ensuring high quality sample maintenance. This skills gap can lead to operational inefficiencies, increased risk of sample degradation, and delays in adopting modern technologies, ultimately limiting the region’s capacity to fully leverage advanced preservation solutions.
Infrastructure Gaps in Cold Chain and Storage Facilities
The reliability of biopreservation in South East Asia is often challenged by uneven cold chain infrastructure, limited storage capacity, and insufficient backup systems. Facilities in certain regions may experience inconsistent temperature control, inadequate monitoring, or disruptions in power supply, all of which compromise sample integrity. These infrastructure constraints hinder long term preservation reliability, restrict scalability, and pose operational challenges for research organizations and healthcare providers seeking dependable storage environments.
Competitive Landscape:
The competitive landscape of the South East Asia biopreservation market is characterized by steady technological advancement and increasing participation from regional and international players. Companies are focusing on strengthening product portfolios with improved cryogenic storage systems, automated sample handling solutions, and optimized preservation media to meet evolving research and clinical needs. Collaboration between industry, research institutes, and healthcare providers is intensifying as organizations work to enhance biobanking capacity and streamline sample management workflows. Competitive differentiation is increasingly driven by innovation, reliability, customization capabilities, and the ability to support scalable preservation infrastructure across diverse applications.
Recent Developments:
- In August 2025, Haier Biomedical formed a strategic partnership with Thailand’s RAM Medical Group to enhance healthcare through pharmacy automation and smart solutions. This collaboration aims to address Southeast Asia's healthcare upgrade needs, utilizing Haier's expertise to tailor solutions for local markets, ultimately benefiting patients and medical tourists.
- In January 2025, Vinmec Tissue Bank become Vietnam's first private tissue bank licensed by the Ministry of Health. Officially operational since February 26, 2020, it focuses on collecting and supplying biological samples for research and treatment, enhancing medical services and international collaborations, backed by advanced infrastructure and trained specialists.
South East Asia Biopreservation Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Product Types Covered | Biopreservation Equipment, Biopreservation Media |
| Applications Covered | Therapeutic Applications, Biobank Applications, Drug Discovery Applications |
| Countries Covered | Indonesia, Thailand, Singapore, Philippines, Vietnam, Malaysia, Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report
The South East Asia biopreservation market size was valued at USD 364.34 Million in 2025.
The South East Asia biopreservation market is expected to grow at a compound annual growth rate of 13.92% from 2026-2034 to reach USD 1,177.35 Million by 2034.
Biopreservation equipment held the largest share of 58% in the market, driven by rising adoption of advanced cryogenic freezers, automated storage systems, and temperature monitoring technologies that support long term sample integrity and expanding research and clinical workflows across the region.
Key factors driving the South East Asia biopreservation market include expanding biobanking activity, rising investment in regenerative medicine, increasing clinical trial volume, and growing adoption of advanced storage technologies that enhance sample quality, operational efficiency, and research scalability.
Major challenges include high costs of advanced preservation equipment, limited technical expertise, and infrastructure gaps in cold chain and storage reliability, which can restrict accessibility, hinder scalability, and affect long term sample integrity across regional facilities.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












