
South Korea Cell Therapy Market Size, Share, Trends and Forecast by Cell Type, Therapy Type, Therapeutic Area, End User, and Region, 2025-2033
South Korea Cell Therapy Market Overview:
The South Korea cell therapy market size reached USD 298.83 Million in 2024. Looking forward, the market is expected to reach USD 1,010.83 Million by 2033, exhibiting a growth rate (CAGR) of 14.50% during 2025-2033. The market is driven by increasing government support for regenerative medicine, rising prevalence of chronic diseases, and strong R&D investments. Expansion of cell manufacturing infrastructure and growing clinical trial activity further influence South Korea cell therapy market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 298.83 Million |
| Market Forecast in 2033 | USD 1,010.83 Million |
| Market Growth Rate 2025-2033 | 14.50% |
South Korea Cell Therapy Market Trends:
Accelerating Clinical Trials and Regulatory Support
South Korea has streamlined regulatory pathways for cell therapy, enabling faster clinical trial approvals and market entry. National initiatives provide funding for regenerative medicine research, supporting both academic institutions and private enterprises. Public-private collaborations have enabled the expansion of trial sites and the acceleration of phase II and III trials targeting oncology, autoimmune, and neurodegenerative diseases. These initiatives facilitate access to novel therapies and attract global partnerships. As the ecosystem matures, the availability of patient populations and well-established clinical infrastructure further attract international investments. This strengthened regulatory and trial landscape underpins South Korea cell therapy market growth. For instance, in June 2025, South Korean researchers at KAIST have pioneered a groundbreaking, non‑toxic approach to cancer treatment. Using an AI-driven tool called BENEIN (Boolean Network Inference), they identified three key genes—MYB, HDAC2, and FOXA2—that sustain malignant behavior in colorectal cancer cells. By silencing these genes simultaneously, tumor cells reverted to normal-like enterocytes in lab cultures and mice, shrinking tumors without chemotherapy or radiation. This work, published in Advanced Science, may mark a major step toward universal, side-effect‑free cancer therapies.
Technological Advancements in Cell Manufacturing
South Korean manufacturers are advancing scalable, GMP-compliant production pipelines for cell-based therapies. Innovations in allogeneic and autologous cell processing, automated bioreactors, and cryopreservation techniques are improving product consistency and reducing costs. Integration of AI-driven analytics optimizes cell quality, yield, and phenotype characterization. These technological advancements promote standardization and facilitate commercial-scale rollouts. Moreover, strategic partnerships between biotech firms and contract development and manufacturing organizations (CDMOs) enhance supply chain resilience. These improvements in manufacturing infrastructure and capabilities represent a key driver of South Korea cell therapy market growth. For instance, in August 2024, SK pharmteco and Rznomics signed an MOU for a multi-year partnership to co-develop and manufacture RNA-based gene therapies. SK pharmteco will support Rznomics with technical expertise and manufacturing capacity to advance products like RZ-001, targeting cancers and neurodegenerative diseases. The collaboration aims to move therapies into late-stage clinical trials and commercialization.
South Korea Cell Therapy Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2025-2033. Our report has categorized the market based on cell type, therapy type, therapeutic area, and end user.
Cell Type Insights:
- Stem Cell
- Bone Marrow
- Blood
- Umbilical Cord-Derived
- Adipose-Derived Stem Cell
- Others
- Non-stem Cell
The report has provided a detailed breakup and analysis of the market based on the cell type. This includes stem cell (bone marrow, blood, umbilical cord-derived, adipose-derived stem cell, and others) and non-stem cell.
Therapy Type Insights:
- Autologous
- Allogeneic
The report has provided a detailed breakup and analysis of the market based on the therapy type. This includes autologous and allogeneic.
Therapeutic Area Insights:
- Malignancies
- Musculoskeletal Disorders
- Autoimmune Disorders
- Dermatology
- Others
The report has provided a detailed breakup and analysis of the market based on the therapeutic area. This includes malignancies, musculoskeletal disorders, autoimmune disorders, dermatology, and others.
End User Insights:
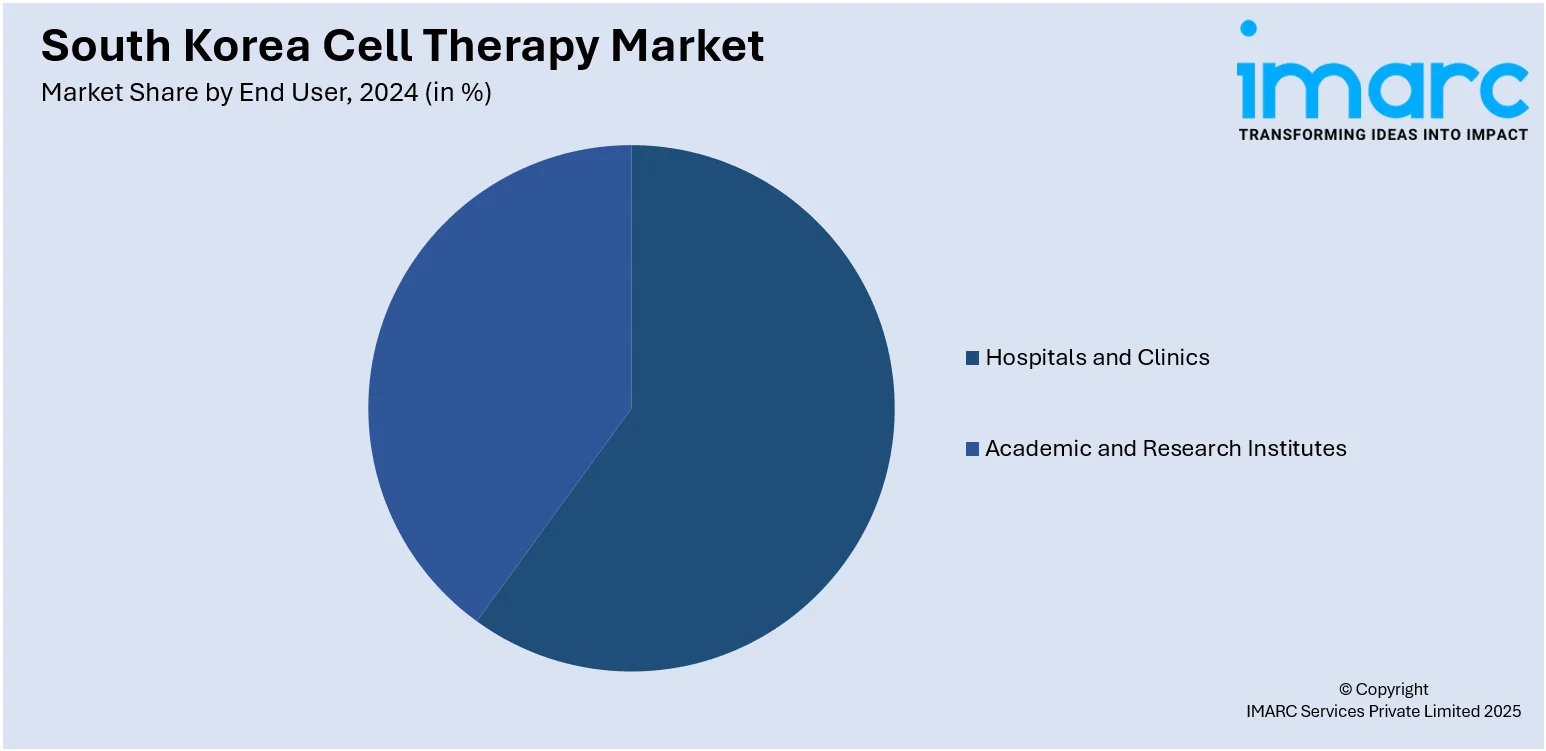
- Hospitals and Clinics
- Academic and Research Institutes
The report has provided a detailed breakup and analysis of the market based on the end user. This includes hospitals and clinics and academic and research institutes.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Cell Therapy Market News:
- In May 2025, Thermo Fisher Scientific launched a Cell and Gene Therapy Vision Centre in South Korea to support biopharmaceutical companies across the entire development pipeline, from discovery to commercialization. The facility offers advanced technologies, regulatory expertise, and hands-on training. It will serve as a regional hub for process optimization and collaboration, enabling faster development of advanced therapies. This strategic expansion reflects the company’s commitment to advancing personalized and regenerative medicine while strengthening its global network of support for cell and gene therapy development.
- In April 2025, South Korea’s DAAN Biotherapeutics signed an exclusive licensing deal with GC Cell to develop CAR-T and CAR-NK therapies targeting a tumor antigen overexpressed in solid cancers. The agreement includes upfront and milestone payments, with GC Cell gaining rights to DAAN’s highly specific antibody for advanced cancer treatment.
South Korea Cell Therapy Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Cell Types Covered |
|
| Therapy Types Covered | Autologous, Allogeneic |
| Therapeutic Areas Covered | Malignancies, Musculoskeletal Disorders, Autoimmune Disorders, Dermatology, Others |
| End Users Covered | Hospitals and Clinics, Academic and Research Institutes |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea cell therapy market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea cell therapy market on the basis of cell type?
- What is the breakup of the South Korea cell therapy market on the basis of therapy type?
- What is the breakup of the South Korea cell therapy market on the basis of therapeutic area?
- What is the breakup of the South Korea cell therapy market on the basis of end user?
- What is the breakup of the South Korea cell therapy market on the basis of region?
- What are the various stages in the value chain of the South Korea cell therapy market?
- What are the key driving factors and challenges in the South Korea cell therapy market?
- What is the structure of the South Korea cell therapy market and who are the key players?
- What is the degree of competition in the South Korea cell therapy market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea cell therapy market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea cell therapy market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea cell therapy industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












