
South Korea Companion Diagnostics Market Size, Share, Trends and Forecast by Product and Service, Technology, Indication, End User, and Region, 2025-2033
South Korea Companion Diagnostics Market Overview:
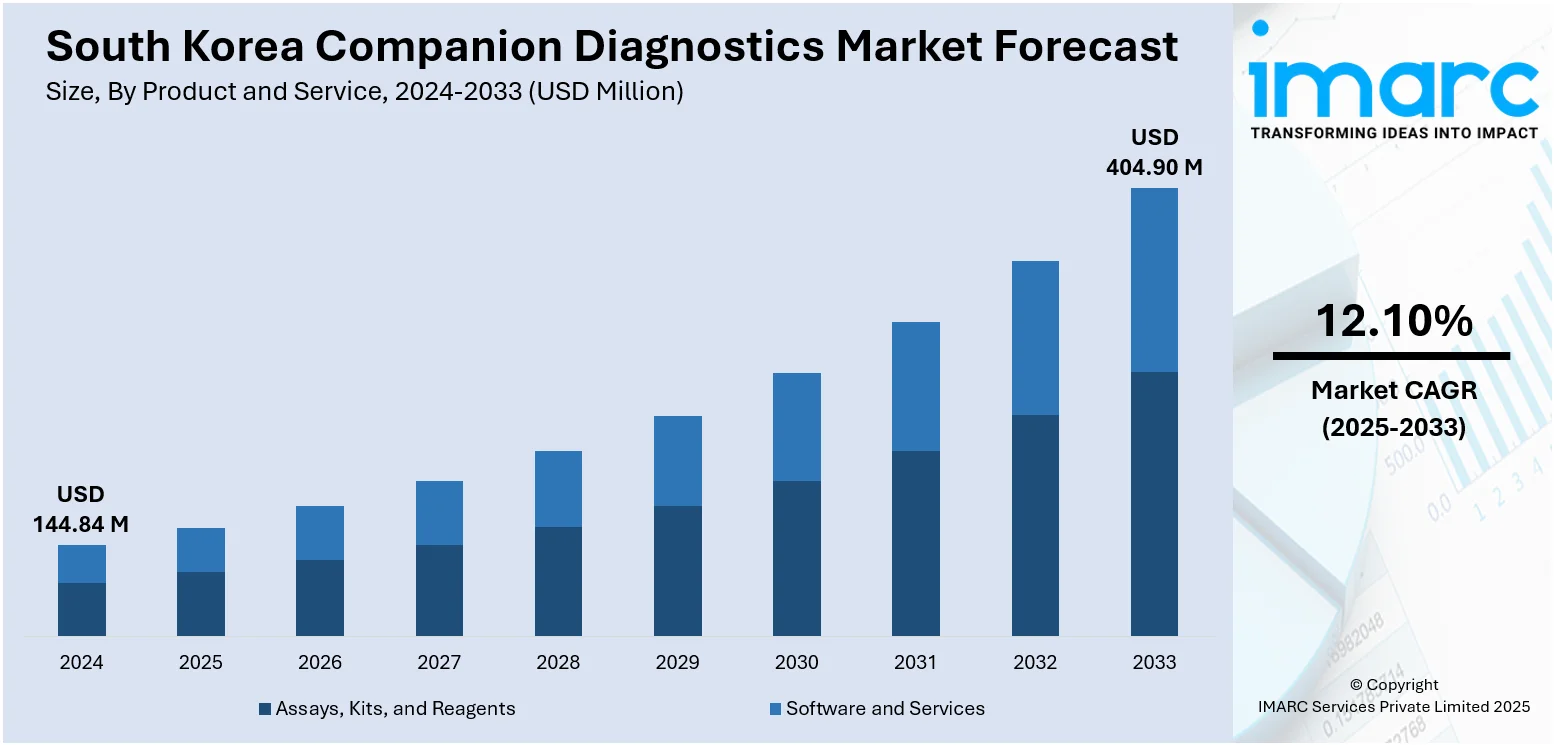
The South Korea companion diagnostics market size reached USD 144.84 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 404.90 Million by 2033, exhibiting a growth rate (CAGR) of 12.10% during 2025-2033. The governing body is supporting the growth of companion diagnostics market through strategic healthcare reforms, subsidies, and investments in biotechnology. Additionally, the rise of telemedicine and digital health solutions, which is enhancing accessibility, enabling remote consultations and diagnostics, and expanding the reach of personalized healthcare services, is increasing the South Korea companion diagnostics market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 144.84 Million |
| Market Forecast in 2033 | USD 404.90 Million |
| Market Growth Rate 2025-2033 | 12.10% |
South Korea Companion Diagnostics Market Trends:
Government Initiatives and Healthcare Reforms
The governing authority in South Korea is pivotal in influencing the domestic companion diagnostics market via strategic healthcare reforms and investments in innovation. Due to a swiftly aging populace and rising healthcare needs, the governing body is emphasizing the incorporating advanced diagnostic technologies into the country’s medical system. To speed up the use of advanced technologies, the governing body is introducing subsidies, favorable reimbursement policies, and cultivated public-private partnerships (PPP). These efforts not only facilitate the regulatory approval process but also enhance the accessibility of companion diagnostics to a broader patient population. Additionally, rising investment in biotechnology and healthcare research, fostering a conducive environment for the creation and commercialization of diagnostic solutions. A significant instance is the declaration in 2024, when the Ministry of Science and ICT disclosed a ₩2.2 trillion ($1.59 billion) allocation for healthcare R&D. This investment focused on sectors, such as mental health, welfare for the elderly and disabled, and fertility treatments, aiming to enhance South Korea’s position in the international bio sector. By supporting these initiatives, the governing authority is advocating for affordable, high-quality healthcare options that directly correspond with the growing need for personalized, precision-focused diagnostics. These initiatives are contributing to the South Korea companion diagnostics market growth, positioning the country as a key contributor to worldwide healthcare innovation.
To get more information on this market, Request Sample
Growth in Telemedicine and Remote Diagnostics
The emergence of telehealth platforms and digital health solutions is transforming healthcare services in South Korea, allowing companion diagnostics to reach a wider audience. Healthcare providers are progressively delivering remote consultations and diagnostic services, especially for patients in rural or underserved regions. Transitioning to digital health facilitates improved management of patient information and supports remote oversight, offering tailored treatment suggestions grounded in diagnostic outcomes. Integrating companion diagnostics with telemedicine systems allows healthcare professionals to work remotely with patients to analyze test results and modify treatments, improving the overall patient experience. This trend is additionally backed by the robust telemedicine sector in South Korea, which attained USD 1,744.4 million in 2024. With the rise of telehealth services, access to companion diagnostics is significantly increasing, allowing patients to avoid traveling to specialized centers for diagnostic testing or consultations. This accessibility enhances convenience and also facilitates the broader utilization of companion diagnostics, accelerating their adoption nationwide. As telemedicine evolves into an essential element of healthcare systems, particularly in rural regions, companion diagnostics are becoming more important, aiding the nation’s shift toward more tailored and efficient healthcare approaches.
South Korea Companion Diagnostics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2025-2033. Our report has categorized the market based on product and service, technology, indication, and end user.
Product and Service Insights:
- Assays, Kits, and Reagents
- Software and Services
The report has provided a detailed breakup and analysis of the market based on the product and service. This includes assays, kits, and reagents and software and services.
Technology Insights:
- Immunohistochemistry (IHC)
- Polymerase Chain Reaction (PCR)
- In-situ Hybridization (ISH)
- Real-time PCR (RT-PCR)
- Gene Sequencing
- Others
A detailed breakup and analysis of the market based on the technology have also been provided in the report. This includes immunohistochemistry (IHC), polymerase chain reaction (PCR), in-situ hybridization (ISH), real-time PCR (RT-PCR), gene sequencing, and others.
Indication Insights:
- Cancer
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Gastric Cancer
- Melanoma
- Others
- Neurological Diseases
- Infectious Diseases
- Cardiovascular Diseases
- Others
The report has provided a detailed breakup and analysis of the market based on the indication. This includes cancer (lung cancer, breast cancer, colorectal cancer, gastric cancer, melanoma, and others), neurological diseases, infectious diseases, cardiovascular diseases, and others.
End User Insights:
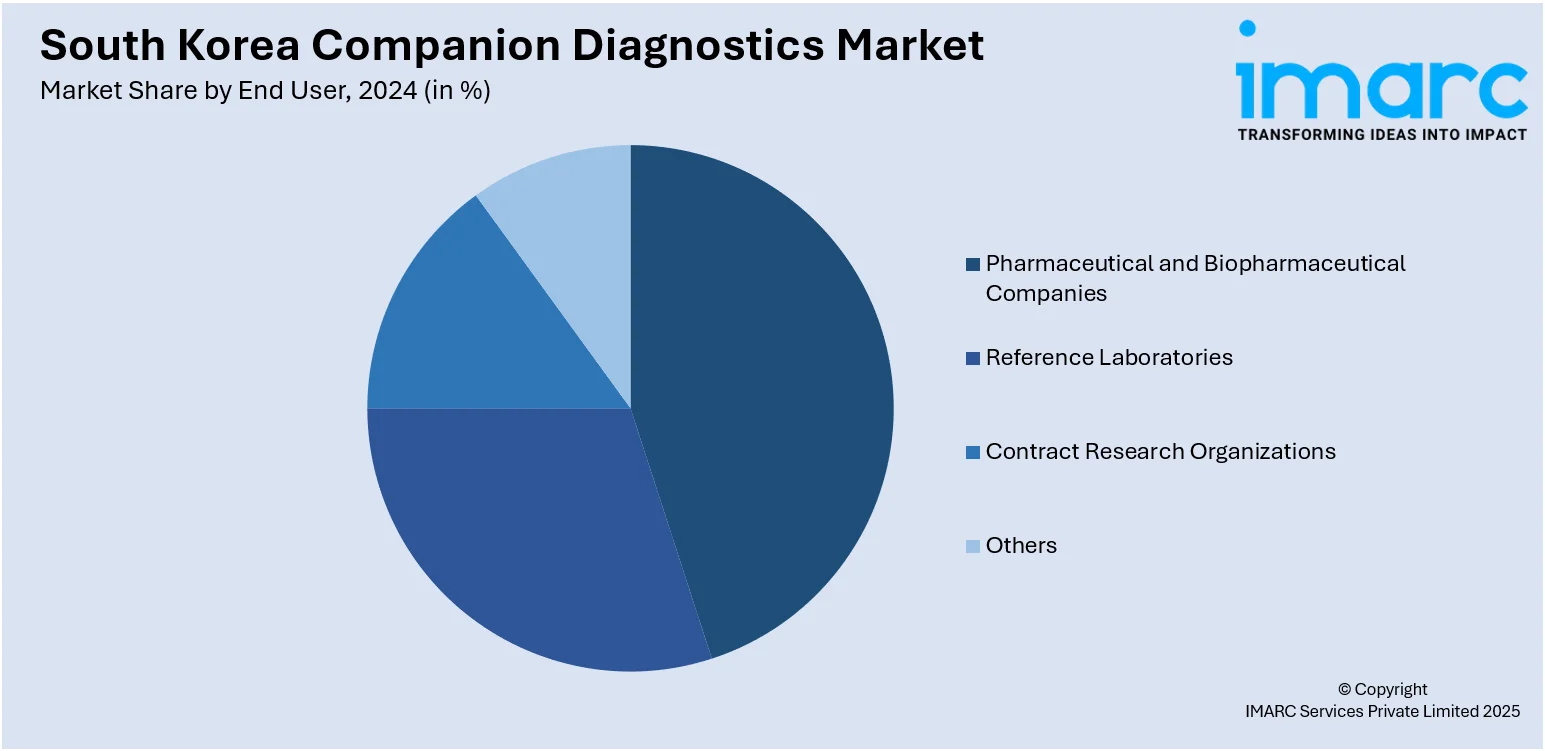
- Pharmaceutical and Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
- Others
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes pharmaceutical and biopharmaceutical companies, reference laboratories, contract research organizations, and others.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Companion Diagnostics Market News:
- In February 2025, HLB PANAGENE received approval in South Korea for its PCR-based companion diagnostic device, PANAMutyper™ ROS1, used to detect ROS1 mutations in non-small cell lung cancer patients. This enables faster, more efficient prescription of targeted therapy such as Pfizer’s Xalkori. It is the first domestically developed ROS1 companion diagnostic approved in Korea.
- In September 2024, ABION and Deep Bio signed an MOU in Seoul, South Korea to collaborate on AI-driven companion diagnostics and cancer clinical trials. The partnership integrated Deep Bio’s AI-powered pathology tools into ABION’s drug pipeline to enhance precision in patient selection and accelerate trial efficiency. This supported ABION’s ongoing combination trials in lung cancer therapy.
South Korea Companion Diagnostics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Product and Services Covered | Assays, Kits, and Reagents, Software and Services |
| Technologies Covered | Immunohistochemistry (IHC), Polymerase Chain Reaction (PCR), In-situ Hybridization (ISH), Real-time PCR (RT-PCR), Gene Sequencing, Others |
| Indications Covered |
|
| End Users Covered | Pharmaceutical and Biopharmaceutical Companies, Reference Laboratories, Contract Research Organizations, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea companion diagnostics market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea companion diagnostics market on the basis of product and service?
- What is the breakup of the South Korea companion diagnostics market on the basis of technology?
- What is the breakup of the South Korea companion diagnostics market on the basis of indication?
- What is the breakup of the South Korea companion diagnostics market on the basis of end user?
- What is the breakup of the South Korea companion diagnostics market on the basis of region?
- What are the various stages in the value chain of the South Korea companion diagnostics market?
- What are the key driving factors and challenges in the South Korea companion diagnostics market?
- What is the structure of the South Korea companion diagnostics market and who are the key players?
- What is the degree of competition in the South Korea companion diagnostics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea companion diagnostics market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea companion diagnostics market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea companion diagnostics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












