
South Korea Digital Therapeutics Market Size, Share, Trends and Forecast by Application, End Use, and Region, 2026-2034
South Korea Digital Therapeutics Market Size Overview:
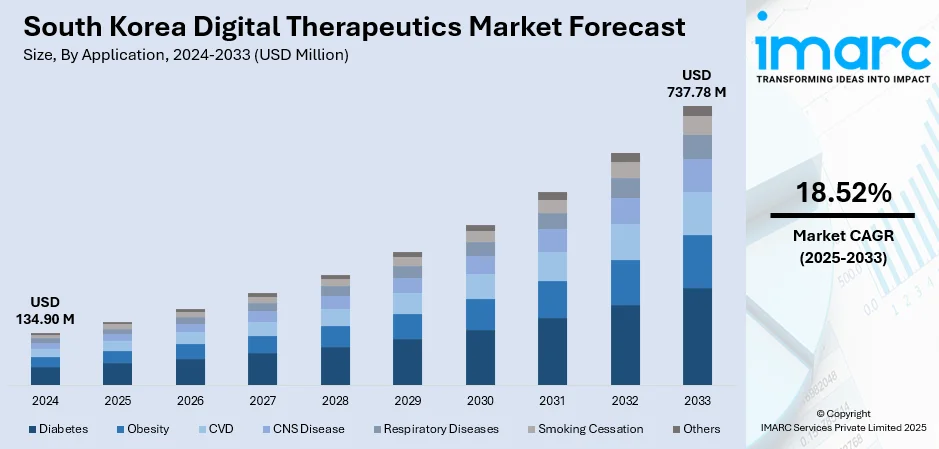
The South Korea digital therapeutics market size reached USD 134.90 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 737.78 Million by 2034, exhibiting a growth rate (CAGR) of 18.52% during 2026-2034. At present, the government of South Korea is pushing digital health innovation aggressively through focused policies and strategic investments. Moreover, the country is facing an expanding burden of chronic diseases and mental illnesses, which is driving the demand for scalable and efficacious therapeutic solutions. This, along with the advanced digital infrastructure and broad tech literacy among the masses, is expanding the South Korea digital therapeutics market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025
|
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 134.90 Million |
| Market Forecast in 2034 | USD 737.78 Million |
| Market Growth Rate 2026-2034 | 18.52% |
South Korea Digital Therapeutics Market Trends:
Government Policies and Regulation Support
The government of South Korea is pushing digital health innovation aggressively through focused policies and strategic investments. By initiating programs under such schemes as the Digital New Deal, the authorities are giving high priority to incorporating advanced technologies into the healthcare system. Regulatory institutions like the Ministry of Food and Drug Safety (MFDS) are launching simplified approval mechanisms directly for digital therapeutics, which shorten development periods and improve market entry. The government is also supporting pilot initiatives and promoting public-private collaborations to pilot and scale digital therapeutic offerings. Consequently, local entrepreneurs and international firms are coming into the South Korean market, attracted by regulatory clarity and financial incentives. This continuous government engagement is developing an ecosystem that is supportive of innovation, clinical uptake is growing, and the broader digital therapeutics space is becoming more dynamic and mature. In January 2024, South Korea implemented the Digital Medical Products Act (DMPA), an important law aimed at overseeing the expanding area of digital health technologies, such as artificial intelligence (AI)-powered devices, digital therapeutics, and health-related applications. The DMPA, being the initial all-encompassing legislation dedicated entirely to digital medical products, signified a significant advancement in securing the safety, efficacy, and creativity of digital healthcare solutions.
To get more information on this market, Request Sample
Increasing Burden of Chronic and Mental Health Disorders
South Korea is facing an expanding burden of chronic diseases and mental illnesses, and this is driving the demand for scalable and efficacious therapeutic solutions. Aging population, sedentary habits, and changes in diet are also contributing towards the increase in diabetes, hypertension, and cardiovascular conditions, thereby impelling the South Korea digital therapeutics market growth. Mental health conditions like depression, anxiety, and insomnia are also on the rise, especially among young people. The patients are looking for convenient and non-invasive treatment options, and digital therapeutics are addressing this by offering customized, evidence-based treatments via mobile apps and connected platforms. Healthcare professionals are embracing these technologies to conduct long-term patient interaction and track treatment results from a distance. Furthermore, the social stigma against conventional mental health therapy is compelling more people to resort to digital means that provide anonymity and convenience. As these healthcare issues continue escalating, digital therapeutics have become ever-more vital for enabling disease management and enhancing quality of life.
Technological Progress and High Digital Literacy
South Korea is using its advanced digital infrastructure and broad tech literacy to enable the accelerated uptake of digital therapeutics. With one of the highest smartphone penetrations and lightning-fast internet connections, the nation is lending an enabling environment for mobile health solutions. People are exhibiting high degrees of digital literacy and willingness to embrace new technology, making them perfect candidates for digital therapeutic solutions. Meanwhile, technology firms based in their region are creating and releasing sophisticated tools, such as AI-based diagnostics, smart wearables, and cloud-based therapy platforms. These technological solutions are constantly improving the user experience and capabilities of digital therapeutics. Data protection protocols and cybersecurity systems are being fortified to comply with privacy requirements, facilitating more trust in digital health products. With digital technologies always developing and becoming more integrated into healthcare services, South Korea is emerging as the leader in the creation and use of next-generation therapeutic products. In 2024, Asan Medical Centre (AMC) in South Korea started recommending VIVID Brain, a digital therapy designed for individuals with visual deficits resulting from a stroke. A 12-week therapy plan requires patients to utilize a virtual reality gadget and a mobile app. VIVID Brain conducts a visual perception assessment to determine the best training site. Moreover, the device can personalize treatment by automatically modifying the difficulty level based on each patient's progress in training.
South Korea Digital Therapeutics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2026-2034. Our report has categorized the market based on application and end use.
Application Insights:
- Diabetes
- Obesity
- CVD
- CNS Disease
- Respiratory Diseases
- Smoking Cessation
- Others
The report has provided a detailed breakup and analysis of the market based on the application. This includes diabetes, obesity, CVD, CNS disease, respiratory diseases, smoking cessation, and others.
End Use Insights:
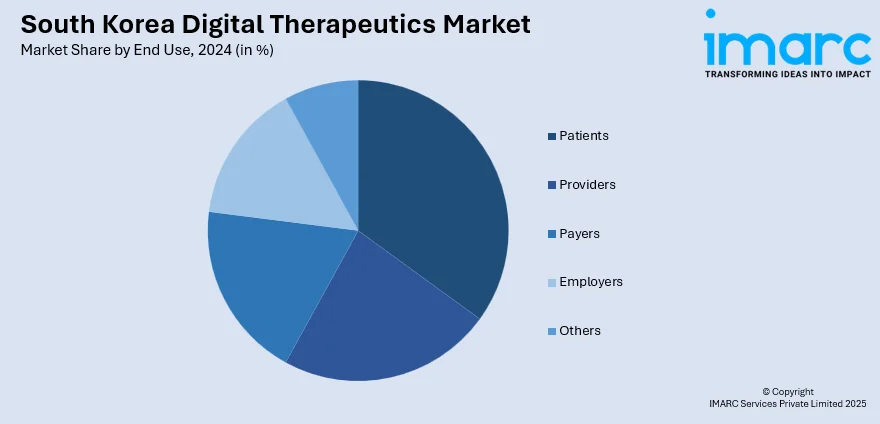
- Patients
- Providers
- Payers
- Employers
- Others
A detailed breakup and analysis of the market based on the end use have also been provided in the report. This includes patients, providers, payers, employers, and others.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Digital Therapeutics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Applications Covered | Diabetes, Obesity, CVD, CNS Disease, Respiratory Diseases, Smoking Cessation, Others |
| End Uses Covered | Patients, Providers, Payers, Employers, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea digital therapeutics market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea digital therapeutics market on the basis of application?
- What is the breakup of the South Korea digital therapeutics market on the basis of end use?
- What is the breakup of the South Korea digital therapeutics market on the basis of region?
- What are the various stages in the value chain of the South Korea digital therapeutics market?
- What are the key driving factors and challenges in the South Korea digital therapeutics market?
- What is the structure of the South Korea digital therapeutics market and who are the key players?
- What is the degree of competition in the South Korea digital therapeutics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea digital therapeutics market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea digital therapeutics market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea digital therapeutics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












