
South Korea Genetic Testing Market Size, Share, Trends and Forecast by Type, Technology, Application, and Region, 2025-2033
South Korea Genetic Testing Market Overview:
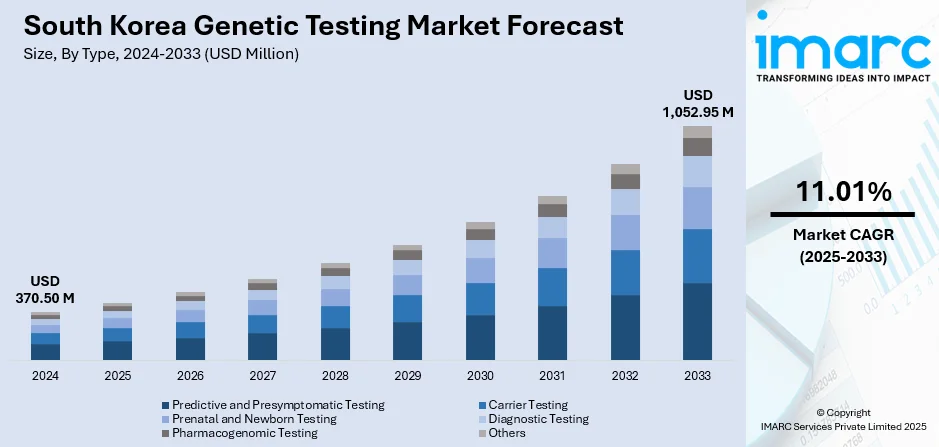
The South Korea genetic testing market size reached USD 370.50 Million in 2024. The market is projected to reach USD 1,052.95 Million by 2033, exhibiting a growth rate (CAGR) of 11.01% during 2025-2033. The market is propelled by robust government funding and national precision medicine strategies, including a new bio transformation initiative. Enhanced awareness of hereditary and rare disorders, along with rising direct to consumer interest, fuels demand. Technological advances, especially next generation sequencing, molecular diagnostics, and AI integration, are reducing costs and improving access. A strong healthcare infrastructure and active public private collaboration further support growth, contributing to an increasing South Korea genetic testing market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 370.50 Million |
| Market Forecast in 2033 | USD 1,052.95 Million |
| Market Growth Rate 2025-2033 | 11.01% |
South Korea Genetic Testing Market Trends:
Preventive and Wellness Expansion
In February 2025, the Ministry of Health and Welfare reported that over million individuals had accessed government-sponsored genetic panels through public clinics. That milestone underscores a major push toward preventive healthcare: genetic testing is increasingly integrated into routine wellness checks. These panels now regularly assess traits related to medication response, nutrient metabolism, and carrier risk for common conditions, empowering individuals to make informed lifestyle and health decisions. Physicians are also integrating genetic results into broader care plans, recommending preventive actions based on personalized data. At the same time, clinical labs are enhancing user experience by reducing processing times and delivering clear, actionable reports. As more of the public recognizes the value of genetic insights, demand extends beyond rare disease screening, positioning genetic services as a regular part of everyday medicine. This evolving mindset signals deep integration of genetic testing into healthcare services, and it stands out as a significant catalyst for long-term South Korea genetic testing market growth.
To get more information on this market, Request Sample
Consumer-Centric Direct Testing
In July 2025, The Korea Times reported that policymakers began considering guidelines requiring teenagers and their guardians to provide dual consent before using direct-to-consumer genetic kits. This discussion reflects a broader trend: households are increasingly ordering at‑home test kits focused on ancestry, dietary predispositions, and wellness traits. The appeal is clear sample collection can be done in the comfort of one’s home, results arrive digitally and privately, and the experience is fast and user-friendly. Early surveys published in national media show many users reporting behavioral changes inspired by test results, in fitness or dietary routines. In response, service providers are expanding their test panels and refining mobile dashboards, offering tiered services that include pharmacogenetic and nutrition-linked modules. This marks a shift toward a more consumer-driven model that removes traditional clinical gatekeeping and enhances the user journey. As adoption grows among younger, tech-savvy demographics, this democratization of genetic data is transforming how services are delivered. The rise of direct-to-consumer kits is reshaping delivery models and stands as a defining element of evolving South Korea genetic testing market trends.
Clinical Integration and Precision Medicine
The National Health Insurance Service reported a dramatic increase in genetic test application in clinical practice, especially in precision medicine and oncology, in May 2025. Genetic panels are used on a routine basis in diagnosing and treating various forms of cancer, which helps physicians tailor therapies based on the genetic profile of patients. Hospitals have streamlined genetic test result review workflows and processes through the implementation of improved ethical frameworks and data-sharing protocols. Increased insurance coverage of genetic testing has offered broader access to more patients, reducing costs and ensuring adoption even at the initial stages of care. These advances have led to more precise treatment, improved patient outcomes, and reduced unecessary procedures. In addition, clinical genetic testing promotes research towards better disease mechanisms and novel therapy development. Rising integration of genetic testing within the healthcare infrastructure is one of the key reasons for the pace of the South Korea.
South Korea Genetic Testing Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2025-2033. Our report has categorized the market based on type, technology, and application.
Type Insights:
- Predictive and Presymptomatic Testing
- Carrier Testing
- Prenatal and Newborn Testing
- Diagnostic Testing
- Pharmacogenomic Testing
- Others
The report has provided a detailed breakup and analysis of the market based on the type. This includes predictive and presymptomatic testing, carrier testing, prenatal and newborn testing, diagnostic testing, pharmacogenomic testing, and others.
Technology Insights:
- Cytogenetic Testing and Chromosome Analysis
- Biochemical Testing
- Molecular Testing
- DNA Sequencing
- Others
A detailed breakup and analysis of the market based on the technology have also been provided in the report. This includes cytogenetic testing and chromosome analysis, biochemical testing, and molecular testing (DNA sequencing and others).
Application Insights:
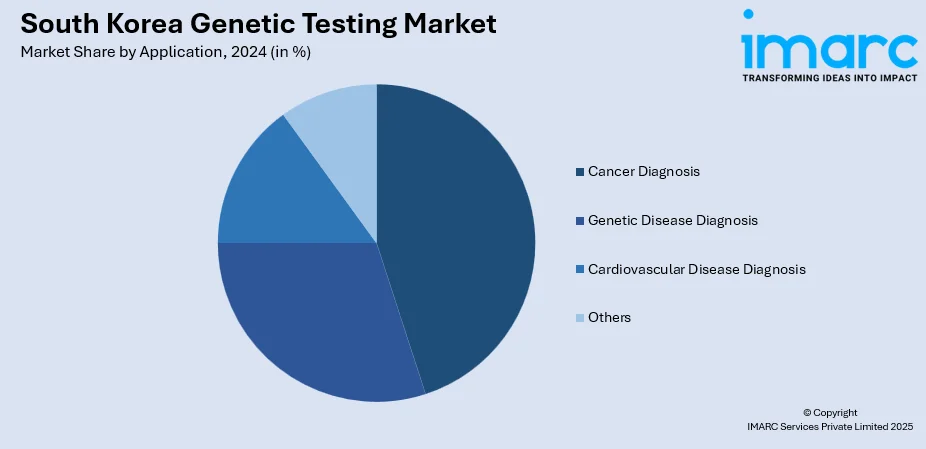
- Cancer Diagnosis
- Genetic Disease Diagnosis
- Cardiovascular Disease Diagnosis
- Others
The report has provided a detailed breakup and analysis of the market based on application. This includes cancer diagnosis, genetic disease diagnosis, cardiovascular disease diagnosis, and others.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include the Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Genetic Testing Market News:
- February 2025: South Korean genetic analysis company 3billion has partnered with AstraZeneca Korea to develop an AI-powered system for faster diagnosis of atypical Hemolytic Uremic Syndrome (aHUS), a rare and serious disease. This collaboration aims to shorten the diagnostic process significantly by analyzing millions of genetic variations with high accuracy. AstraZeneca Korea will provide its approved treatments for aHUS, supported by national health insurance. This partnership marks a major step forward in improving diagnosis and treatment options for aHUS patients in South Korea.
- December 2024: GC Genome, a South Korean clinical genomics company, has applied for a preliminary review to list on the KOSDAQ market. The company recently received a high technology evaluation rating, supporting its plans for an initial public offering. GC Genome provides a wide range of genetic testing services, including prenatal and cancer diagnostics, to numerous hospitals and clinics across South Korea. Following the listing, the company aims to strengthen its presence in North America, expanding its global footprint.
South Korea Genetic Testing Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Types Covered | Predictive and Presymptomatic Testing, Carrier Testing, Prenatal and Newborn Testing, Diagnostic Testing, Pharmacogenomic Testing, Others |
| Technologies Covered |
|
| Applications Covered | Cancer Diagnosis, Genetic Disease Diagnosis, Cardiovascular Disease Diagnosis, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea genetic testing market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea genetic testing market on the basis of type?
- What is the breakup of the South Korea genetic testing market on the basis of technology?
- What is the breakup of the South Korea genetic testing market on the basis of application?
- What is the breakup of the South Korea genetic testing market on the basis of region?
- What are the various stages in the value chain of the South Korea genetic testing market?
- What are the key driving factors and challenges in the South Korea genetic testing market?
- What is the structure of the South Korea genetic testing market and who are the key players?
- What is the degree of competition in the South Korea genetic testing market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea genetic testing market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea genetic testing market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea genetic testing industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












