
South Korea Molecular Diagnostics Market Size, Share, Trends and Forecast by Product, Technology, Application, End User, and Region, 2025-2033
South Korea Molecular Diagnostics Market Overview:
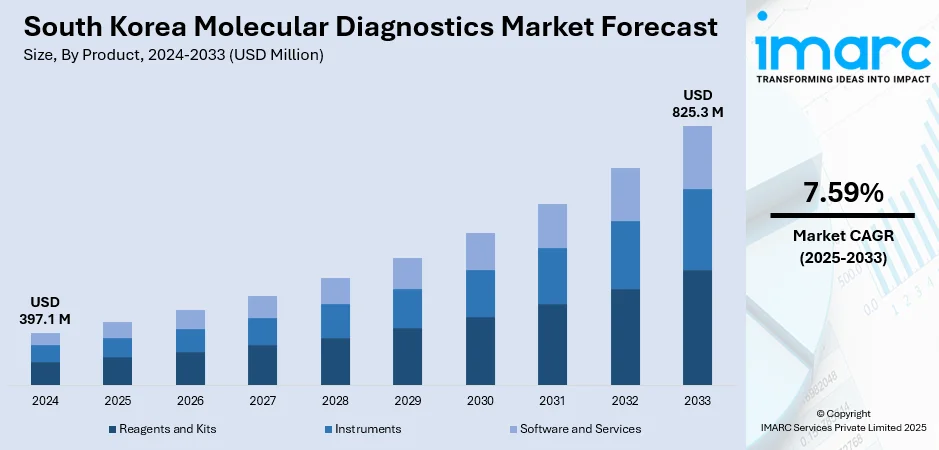
The South Korea molecular diagnostics market size reached USD 397.1 Million in 2024. The market is projected to reach USD 825.3 Million by 2033, exhibiting a growth rate (CAGR) of 7.59% during 2025-2033. The market is expanding due to the increasing adoption of precision medicine, rising cancer cases, and demand for advanced diagnostic tools. Additionally, strong investment in biomarker research and digital health technologies continues to support South Korea molecular diagnostics market share across clinical laboratories, hospitals, and research institutions.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 397.1 Million |
| Market Forecast in 2033 | USD 825.3 Million |
| Market Growth Rate 2025-2033 | 7.59% |
South Korea Molecular Diagnostics Market Trends:
Rising Focus on Personalized Cancer Diagnostics
The South Korea molecular diagnostics market growth is being propelled by the increasing demand for personalized medicine, particularly in oncology. As cancer prevalence continues to rise, healthcare providers and diagnostic companies are focusing on solutions that enable early detection and precise identification of genetic mutations. These advances allow for targeted therapies and reduce the risk of ineffective treatments, improving both patient outcomes and healthcare efficiency. Precision diagnostics are becoming a foundational element in clinical decision-making, especially as payers and providers emphasize value-based care. The push toward integrating biomarker-based diagnostics into routine cancer treatment is reshaping diagnostic protocols nationwide. In October 2024, Ngenebio launched the Oncoaccupanel RNA diagnostic panel in South Korea for detecting oncogenic fusion genes in solid tumors. This RNA-based diagnostic panel supports the identification of fusion genes critical in cancer progression and therapy selection. Its adoption underscores a growing national focus on personalized diagnostic tools that enhance accuracy and therapeutic alignment. The integration of such advanced panels into oncology workflows reflects a larger trend of investing in molecular-level insights for better patient management. With government backing and growing hospital infrastructure, South Korea is rapidly becoming a hub for innovative cancer diagnostics, reinforcing the expansion and depth of its molecular diagnostics capabilities in real-world clinical settings.
To get more information on this market, Request Sample
Technology Integration and Strategic Partnerships
The strategic alignment of biotechnology and digital platforms through collaborative partnerships is positively impacting the market expansion. Diagnostic innovation increasingly depends on combining high-quality biomarker research with advanced data analytics and automation to support fast, reliable testing services. These collaborations are not only improving diagnostic precision but also enabling scalable platforms for broader clinical use. South Korean firms are recognizing the value of integrating hardware, software, and biological research to provide seamless, end-to-end diagnostic solutions that address the full spectrum of patient care. This technology-first mindset is enabling rapid turnaround times, improving workflow efficiency, and ensuring high reliability in test outcomes. In August 2024, Hitachi High-Tech partnered with South Korea’s Gencurix to advance cancer molecular diagnostics by combining biomarker discovery with digital technology. This partnership aimed to build highly accurate, personalized cancer testing services and is part of a broader strategy to meet growing demand for real-time, actionable diagnostic information. The collaboration illustrates a shift toward technology-driven diagnostics, moving beyond traditional testing models. It also highlights how cross-border and cross-sector partnerships are becoming essential in elevating diagnostic service delivery in South Korea. As a result, the market is witnessing stronger integration of molecular diagnostics into hospital systems and research labs, creating new opportunities for growth and innovation.
South Korea Molecular Diagnostics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional level for 2025-2033. Our report has categorized the market based on product, technology, application, and end user.
Product Insights:
- Reagents and Kits
- Instruments
- Software and Services
The report has provided a detailed breakup and analysis of the market based on the product. This includes reagents and kits, instruments, and software and services.
Technology Insights:
- Polymerase Chain Reactions (PCR)
- Hybridization
- DNA Sequencing
- Microarray
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Others
A detailed breakup and analysis of the market based on the technology have also been provided in the report. This includes polymerase chain reactions (PCR), hybridization, DNA sequencing, microarray, isothermal nucleic acid amplification technology (INAAT), and others.
Application Insights:
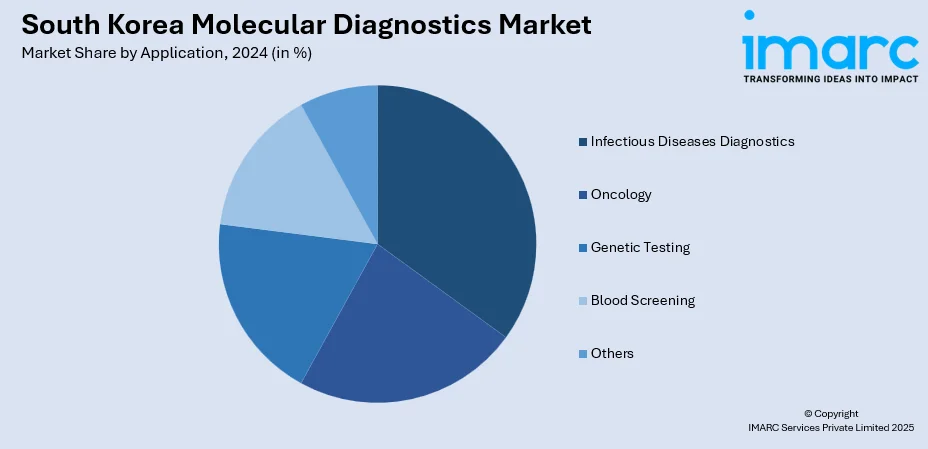
- Infectious Diseases Diagnostics
- Oncology
- Genetic Testing
- Blood Screening
- Others
The report has provided a detailed breakup and analysis of the market based on the application. This includes infectious diseases diagnostics, oncology, genetic testing, blood screening, and others.
End User Insights:
- Hospitals
- Laboratories
- Others
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes hospitals, laboratories, and others.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Molecular Diagnostics Market News:
- July 2025: Seegene launched STAgora, a real-time infectious disease analytics platform integrating diagnostic data with AI and statistical modeling. By enhancing outbreak tracking and predictive capabilities, it advanced South Korea’s molecular diagnostics market, strengthening global public health readiness and accelerating clinical decision-making through real-time insights.
- March 2025: DKSH acquired South Korea-based MDxK, a key provider of molecular diagnostics and scientific solutions. The move expanded DKSH’s presence in South Korea’s life sciences sector, strengthening its molecular diagnostics capabilities and supporting growing demand for advanced healthcare and research technologies in the region.
South Korea Molecular Diagnostics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered | Reagents and Kits, Instruments, Software and Services |
| Technologies Covered | Polymerase Chain Reactions (PCR), Hybridization, DNA Sequencing, Microarray, Isothermal Nucleic Acid Amplification Technology (INAAT), Others |
| Applications Covered | Infectious Diseases Diagnostics, Oncology, Genetic Testing, Blood Screening, Others |
| End Users Covered | Hospitals, Laboratories, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea molecular diagnostics market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea molecular diagnostics market on the basis of product?
- What is the breakup of the South Korea molecular diagnostics market on the basis of technology?
- What is the breakup of the South Korea molecular diagnostics market on the basis of application?
- What is the breakup of the South Korea molecular diagnostics market on the basis of end user?
- What is the breakup of the South Korea molecular diagnostics market on the basis of region?
- What are the various stages in the value chain of the South Korea molecular diagnostics market?
- What are the key driving factors and challenges in the South Korea molecular diagnostics market?
- What is the structure of the South Korea molecular diagnostics market and who are the key players?
- What is the degree of competition in the South Korea molecular diagnostics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea molecular diagnostics market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea molecular diagnostics market.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea molecular diagnostics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive type and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












