
South Korea Pharmaceutical Filtration Market Size, Share, Trends and Forecast by Product, Technique, Application, Scale of Operation, and Region, 2025-2033
South Korea Pharmaceutical Filtration Market Overview:
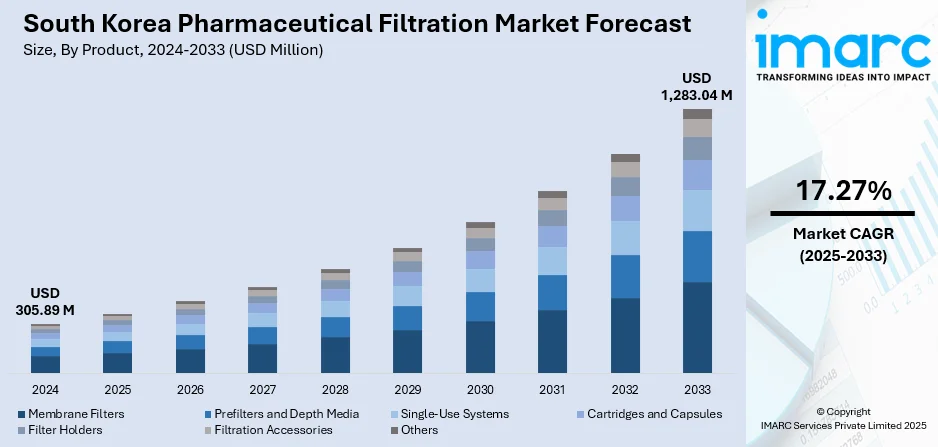
The South Korea pharmaceutical filtration market size reached USD 305.89 Million in 2024. Looking forward, the market is projected to reach USD 1,283.04 Million by 2033, exhibiting a growth rate (CAGR) of 17.27% during 2025-2033. The market is driven by increasing biopharmaceutical R&D investments, stringent regulatory requirements for drug purity and safety, and the rising prevalence of chronic diseases. The expanding biotechnology sector and growing demand for advanced therapeutic solutions also influence the South Korea pharmaceutical filtration market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 305.89 Million |
| Market Forecast in 2033 | USD 1,283.04 Million |
| Market Growth Rate 2025-2033 | 17.27% |
South Korea Pharmaceutical Filtration Market Trends:
Surging Growth of the Biopharmaceutical Industry
South Korea's biopharmaceutical industry is experiencing robust growth, characterized by significant investments in research and development, particularly in areas such as monoclonal antibodies, recombinant proteins, and vaccines. This expansion necessitates highly specialized and efficient filtration technologies for critical processes like protein purification, cell culture media preparation, and viral clearance. As biopharmaceuticals are large, complex molecules, their manufacturing demands stringent purity and safety standards that can only be met with advanced filtration solutions. This burgeoning biopharmaceutical sector is a primary catalyst for the South Korea pharmaceutical filtration market growth, driving demand for innovative and high-performance filters. For instance, in April 2025, SK pharmteco invested $260 Million in a new 135,800 sq ft facility in Sejong, South Korea, to expand its peptide and small-molecule API manufacturing capabilities. The five-story site will feature eight production trains, cGMP kilo labs, a pilot plant, and peptide R&D facilities, supporting both early-stage and commercial pharmaceutical production. This expansion reinforces South Korea’s role in global biopharmaceutical manufacturing and aligns with rising demand for advanced therapies, strengthening the country’s pharmaceutical infrastructure and supply chain resilience.
To get more information on this market, Request Sample
Strict Regulatory Landscape and Quality Assurance
The South Korea pharmaceutical filtration market is profoundly influenced by the nation's stringent regulatory framework, which aligns with global Good Manufacturing Practices (GMP) and other international quality standards. Pharmaceutical manufacturers are mandated to ensure the highest levels of product purity, sterility, and safety, making robust filtration processes indispensable. This regulatory environment compels companies to adopt validated filtration systems capable of consistently removing contaminants, particulates, and microorganisms. The continuous emphasis on quality assurance and compliance with evolving regulatory requirements is a key factor stimulating the South Korea pharmaceutical filtration market growth, as manufacturers invest in reliable and certified filtration technologies. For instance, in June 2024, India, the US, Japan, South Korea, and the EU launched a Biopharmaceutical Alliance at the Bio International Convention 2024 in San Diego to strengthen global drug supply chains. The alliance aims to coordinate bio policies, R&D support, and regulations, while creating a detailed pharmaceutical supply chain map. This initiative responds to drug shortages experienced during the COVID-19 pandemic and seeks to reduce dependence on limited sources of essential raw materials by fostering resilient and sustainable biopharma manufacturing collaboration among member nations.
South Korea Pharmaceutical Filtration Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country/regional level for 2025-2033. Our report has categorized the market based on product, technique, application, and scale of operation.
Product Insights:
- Membrane Filters
- MCE Membrane Filters
- Coated Cellulose Acetate Membrane Filters
- PTFE Membrane Filters
- Nylon Membrane Filters
- PVDF Membrane Filters
- Others
- Prefilters and Depth Media
- Glass Fiber Filters
- PTFE Fiber Filters
- Single-Use Systems
- Cartridges and Capsules
- Filter Holders
- Filtration Accessories
- Others
The report has provided a detailed breakup and analysis of the market based on the product. This includes membrane filters (MCE membrane filters, coated cellulose acetate membrane filters, PTFE membrane filters, nylon membrane filters, PVDF membrane filters, and others), prefilters and depth media (glass fiber filters and PTFE fiber filters), single-use systems, cartridges and capsules, filter holders, filtration accessories, and others.
Technique Insights:
- Microfiltration
- Ultrafiltration
- Crossflow Filtration
- Nanofiltration
- Others
A detailed breakup and analysis of the market based on the technique have also been provided in the report. This includes microfiltration, ultrafiltration, crossflow filtration, nanofiltration, and others.
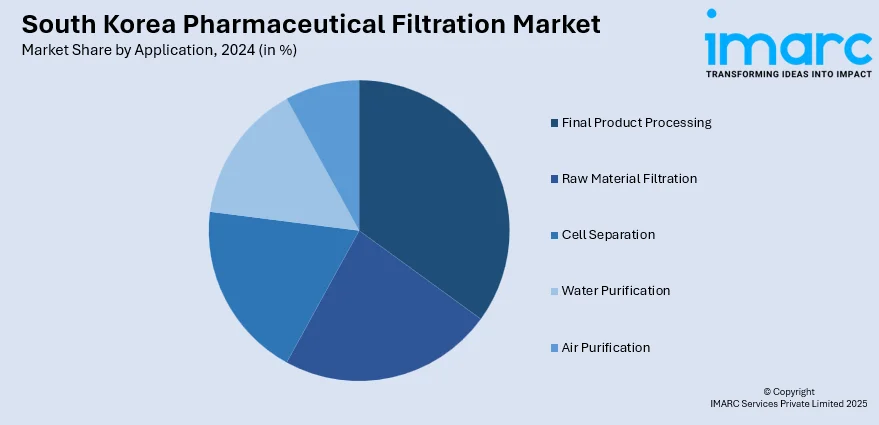
Application Insights:
- Final Product Processing
- Active Pharmaceutical Ingredient Filtration
- Sterile Filtration
- Protein Purification
- Vaccines and Antibody Processing
- Formulation and Filling Solutions
- Viral Clearance
- Raw Material Filtration
- Media Buffer
- Pre-Filtration
- Bioburden Testing
- Cell Separation
- Water Purification
- Air Purification
The report has provided a detailed breakup and analysis of the market based on the application. This includes final product processing (active pharmaceutical ingredient filtration, sterile filtration, protein purification, vaccines and antibody processing, formulation and filling solutions, and viral clearance), raw material filtration (media buffer, pre-filtration, and bioburden testing), cell separation, water purification, and air purification.
Scale of Operation Insights:
- Manufacturing Scale
- Pilot-Scale
- Research and Development Scale
The report has provided a detailed breakup and analysis of the market based on the scale of operation. This includes manufacturing scale, pilot-scale, and research and development scale.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Pharmaceutical Filtration Market News:
- In November 2024, Cytiva opened a new 6,100-square-meter plant in Songdo, Incheon, South Korea, to manufacture sterile-filtration products, strengthening its APAC presence. The facility, expected to be fully operational by 2026, will also expand into virus filtration. Located in the Songdo Bio-cluster, a major biopharma hub, it supports Korea's growing demand for advanced bioprocessing due to rising biologics production. Cytiva’s move aligns with South Korea’s strategic focus on biotechnology and builds on previous partnerships and investments in the region.
South Korea Pharmaceutical Filtration Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered |
|
| Techniques Covered | Microfiltration, Ultrafiltration, Crossflow Filtration, Nanofiltration, Others |
| Applications Covered |
|
| Scales of Operation Covered | Manufacturing Scale, Pilot-Scale, Research and Development Scale |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea pharmaceutical filtration market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea pharmaceutical filtration market on the basis of product?
- What is the breakup of the South Korea pharmaceutical filtration market on the basis of technique?
- What is the breakup of the South Korea pharmaceutical filtration market on the basis of application?
- What is the breakup of the South Korea pharmaceutical filtration market on the basis of scale of operation?
- What is the breakup of the South Korea pharmaceutical filtration market on the basis of region?
- What are the various stages in the value chain of the South Korea pharmaceutical filtration market?
- What are the key driving factors and challenges in the South Korea pharmaceutical filtration market?
- What is the structure of the South Korea pharmaceutical filtration market and who are the key players?
- What is the degree of competition in the South Korea pharmaceutical filtration market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various Korea market segments, historical and current market trends, market forecasts, and dynamics of the South Korea pharmaceutical filtration market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea pharmaceutical filtration market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea pharmaceutical filtration industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












