
South Korea Point of Care Diagnostics Market Size, Share, Trends and Forecast by Product Type, Platform, Prescription Mode, End User, and Region, 2026-2034
South Korea Point of Care Diagnostics Market Overview:
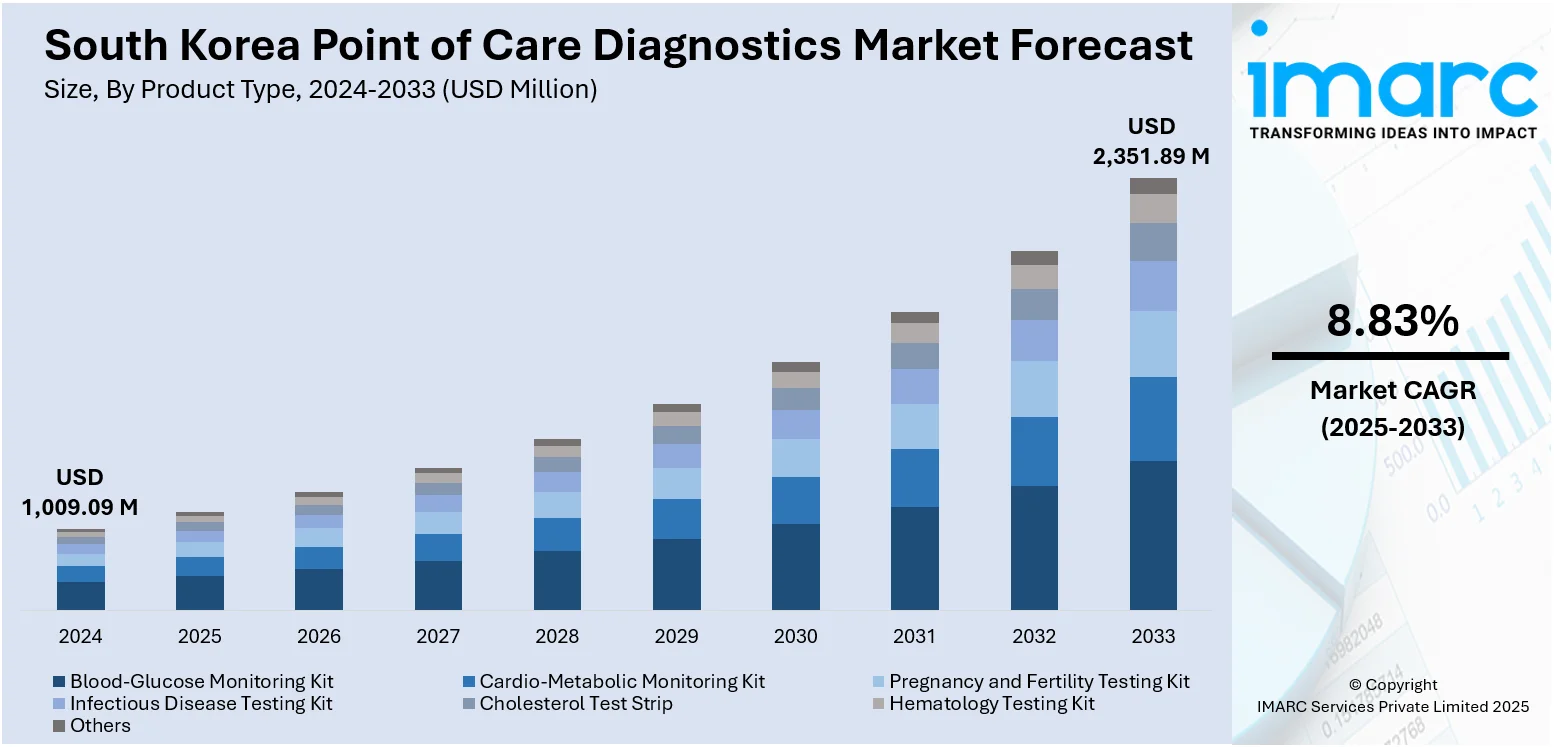
The South Korea point of care diagnostics market size reached USD 1,009.09 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 2,351.89 Million by 2034, exhibiting a growth rate (CAGR) of 8.83% during 2026-2034. South Korea’s aging population, government support for healthcare innovation, and the rise of digital health solutions are driving the point of care diagnostics market. The demand for accessible, efficient diagnostics, supported by favorable policies and telemedicine integration, is further contributing to the expansion of the South Korea point of care diagnostics market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025
|
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 1,009.09 Million |
| Market Forecast in 2034 | USD 2,351.89 Million |
| Market Growth Rate 2026-2034 | 8.83% |
South Korea Point of Care Diagnostics Market Trends:
Aging Population and Increasing Healthcare Needs
South Korea’s swiftly aging demographic is driving the need for point of care (POC) diagnostic solutions. With the rise in the elderly population, the demand for accessible and effective healthcare solutions is becoming more urgent. As per the 2024 Statistics on the Aged, those who are 65 years old and older constitute 19.2% of the population, a percentage anticipated to rise beyond 20% by 2025 and attain 30% by 2036. Elderly individuals, who frequently deal with several chronic illnesses like diabetes, high blood pressure, and kidney failure, require continuous observation. POC diagnostics effectively meet these requirements, providing prompt results that assist in managing these conditions while minimizing the necessity for regular hospital visits. Furthermore, the growing need for fast and easy testing is resulting in an increase in mobile and at-home POC options. The healthcare system's adjustment to enhance long-term care management is further supporting the market growth.
To get more information on this market, Request Sample
Government Support and Healthcare Policy Initiatives
The governing body is playing a critical role in fostering the expansion of the POC diagnostics market via favorable healthcare policies and focused investments. These initiatives comprise regulatory structures that promote innovation in medical technologies and improve the accessibility and effectiveness of healthcare services. For example, in 2024, South Korea pledged US$22 million to Unitaid for global health efforts, which included the creation of advanced diagnostic technologies. This funding targeted enhancing pandemic readiness and increasing local production capacities in low- and middle-income nations, illustrating the government’s dedication to promoting diagnostic innovations. Moreover, the governing authority provides financial support to medical technology startups and partners with educational institutions, fostering the creation of groundbreaking POC solutions. Due to an aging demographic and the increasing demand for effective healthcare, these efforts are anticipated to speed up the implementation of POC diagnostics, enhancing their accessibility and affordability throughout South Korea.
Rise in Healthcare Digitalization and Telemedicine
The continuous expansion of digital health solutions and telemedicine is a major factor bolstering the South Korea point of care diagnostics market growth. As the healthcare sector progressively shifts towards digitalization, there is a higher need for diagnostic tools that can effortlessly connect with telemedicine platforms. POC devices enable patients to conduct tests at home and send the results to healthcare providers remotely, improving the management of chronic conditions and minimizing the necessity for in-person appointments. This integration enhances convenience while also boosting the efficiency of healthcare delivery. As the South Korean telemedicine market approached USD 1,744.4 million in 2024, the need for remote healthcare services is steadily increasing. With the rise of digital health infrastructure, POC diagnostics are becoming an essential part of enhancing virtual healthcare systems, promoting their use, and influencing the future of healthcare nationwide.
South Korea Point of Care Diagnostics Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the country and regional levels for 2026-2034. Our report has categorized the market based on product type, platform, prescription mode, and end user.
Product Type Insights:
- Blood-Glucose Monitoring Kit
- Cardio-Metabolic Monitoring Kit
- Pregnancy and Fertility Testing Kit
- Infectious Disease Testing Kit
- Cholesterol Test Strip
- Hematology Testing Kit
- Others
The report has provided a detailed breakup and analysis of the market based on the product type. This includes blood-glucose monitoring kit, cardio-metabolic monitoring kit, pregnancy and fertility testing kit, infectious disease testing kit, cholesterol test strip, hematology testing kit, and others.
Platform Insights:
- Lateral Flow Assays
- Dipsticks
- Microfluidics
- Molecular Diagnostics
- Immunoassays
A detailed breakup and analysis of the market based on the platform have also been provided in the report. This includes lateral flow assays, dipsticks, microfluidics, molecular diagnostics, and immunoassays.
Prescription Mode Insights:
- Prescription-based Testing
- OTC Testing
The report has provided a detailed breakup and analysis of the market based on the prescription mode. This includes prescription-based testing and OTC testing.
End User Insights:
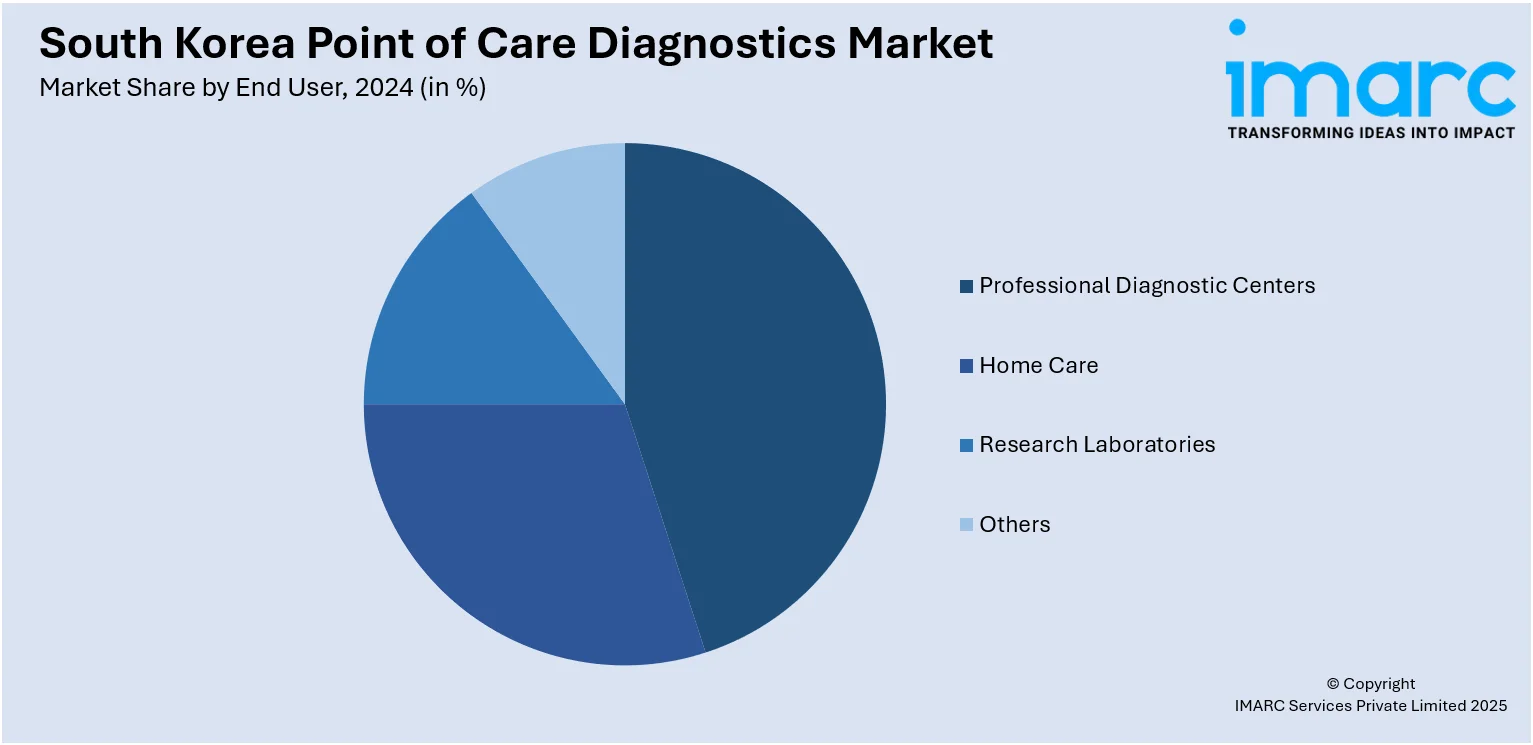
- Professional Diagnostic Centers
- Home Care
- Research Laboratories
- Others
A detailed breakup and analysis of the market based on the end user have also been provided in the report. This includes professional diagnostic centers, home care, research laboratories, and others.
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
The report has also provided a comprehensive analysis of all the major regional markets, which include Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), and others.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
South Korea Point of Care Diagnostics Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Product Types Covered | Blood-Glucose Monitoring Kit, Cardio-Metabolic Monitoring Kit, Pregnancy and Fertility Testing Kit, Infectious Disease Testing Kit, Cholesterol Test Strip, Hematology Testing Kit, Others |
| Platforms Covered | Lateral Flow Assays, Dipsticks, Microfluidics, Molecular Diagnostics, Immunoassays |
| Prescription Modes Covered | Prescription-based Testing, OTC Testing |
| End Users Covered | Professional Diagnostic Centers, Home Care, Research Laboratories, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the South Korea point of care diagnostics market performed so far and how will it perform in the coming years?
- What is the breakup of the South Korea point of care diagnostics market on the basis of product type?
- What is the breakup of the South Korea point of care diagnostics market on the basis of platform?
- What is the breakup of the South Korea point of care diagnostics market on the basis of prescription mode?
- What is the breakup of the South Korea point of care diagnostics market on the basis of end user?
- What is the breakup of the South Korea point of care diagnostics market on the basis of region?
- What are the various stages in the value chain of the South Korea point of care diagnostics market?
- What are the key driving factors and challenges in the South Korea point of care diagnostics market?
- What is the structure of the South Korea point of care diagnostics market and who are the key players?
- What is the degree of competition in the South Korea point of care diagnostics market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea point of care diagnostics market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the South Korea point of care diagnostics market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea point of care diagnostics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












