
Amyotrophic Lateral Sclerosis Treatment Market Size, Share, Trends and Forecast by ALS Type, Drug Type, Diagnosis Type, Treatment, Distribution Channel, and Region, 2025-2033
Amyotrophic Lateral Sclerosis Treatment Market Size and Share:
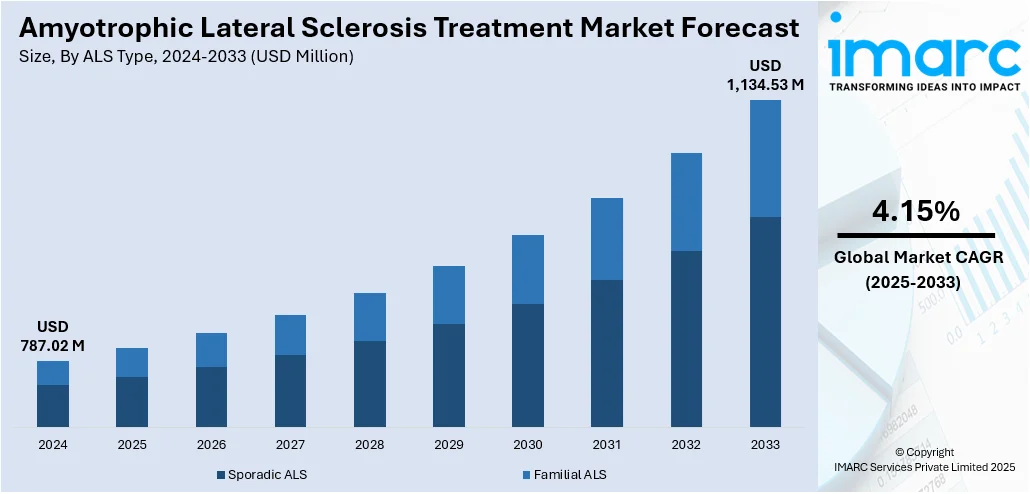
The global amyotrophic lateral sclerosis treatment market size was valued at USD 787.02 Million in 2024. Looking forward, IMARC Group estimates the market to reach USD 1,134.53 Million by 2033, exhibiting a CAGR of 4.15% during 2025-2033. North America currently dominates the market, holding a significant market share of 56.2% in 2024. The region leads the market due to robust healthcare infrastructure, extensive research funding, and high disease awareness. The region also benefits from strong regulatory support for clinical trials, access to cutting-edge therapies, and well-established reimbursement policies, contributing to its dominance in the amyotrophic lateral sclerosis treatment market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 787.02 Million |
|
Market Forecast in 2033
|
USD 1,134.53 Million |
| Market Growth Rate 2025-2033 | 4.15% |
The growth of the market is primarily driven by the increasing demand for innovative therapies due to the rising incidence of ALS worldwide. The expansion of personalized medicine, coupled with advancements in genetic and biomarker research, allows for more targeted and effective treatments. A recent example of such innovation is the MIROCALS clinical trial, led by Queen Mary University of London, which showed promising results by adding low-dose interleukin-2 (IL2LD) to standard ALS treatment, potentially slowing functional decline and extending lifespan. This trial highlights the ongoing development of novel therapies in ALS treatment. Furthermore, government initiatives promoting the development of rare disease treatments and orphan drug designations are encouraging research in ALS. Increased investment from both public and private sectors, alongside collaborations between biotech and pharmaceutical companies, is accelerating the discovery of novel therapies, thus driving market expansion. Enhanced awareness of ALS and its devastating impact also contributes to growing demand for treatment options.
To get more information on this market, Request Sample
In the United States, the amyotrophic lateral sclerosis treatment market growth is bolstered by strong research funding from both governmental agencies such as the National Institutes of Health (NIH) and private sectors. The rise in ALS awareness campaigns and support groups has led to higher patient advocacy and demand for treatment options. Moreover, the development of cutting-edge gene therapies and cell-based treatments, along with regulatory support from the U.S. Food and Drug Administration (FDA), encourages innovation. The presence of leading biotech and pharmaceutical firms in the U.S. fosters an environment conducive to accelerating clinical trials and improving drug availability, further propelling expansion.
Amyotrophic Lateral Sclerosis Treatment Market Trends:
Rising Incidence of Neurological Disorders
The increasing prevalence of neurological disorders is a key amyotrophic lateral sclerosis treatment market trend. According to a 2023 report by the U.S. National Institutes of Health (NIH), around 15% of individuals globally are affected by neurological conditions, which are the leading cause of physical and cognitive disabilities. This rising incidence is creating a greater need for advanced ALS therapies and interventions. The growing recognition of neurological diseases as a significant public health concern is spurring increased demand for specialized treatments, raising awareness, and fostering medical innovations to address the challenges associated with these disorders. This trend is expected to continue as global healthcare systems adapt to the increasing burden of neurodegenerative diseases.
Expanding Geriatric Population Driving Demand for ALS Treatments
The aging population worldwide is playing a significant role in the growth of the ALS treatment market. By 2050, the World Health Organization (WHO) predicts that the number of people aged 60 and above will reach 2.1 billion, with individuals aged 80 years and over expected to grow to 426 million. As older individuals are more susceptible to neurodegenerative diseases like ALS, this demographic shift will increase the demand for specialized treatments, healthcare services, and early diagnosis initiatives. The expansion of the geriatric population is a key factor influencing the positive amyotrophic lateral sclerosis treatment market outlook, as healthcare providers focus on addressing the needs of an aging population with heightened vulnerability to ALS and similar disorders.
Breakthrough Innovations in ALS Treatment
Advancements in ALS treatment are significantly shaping the market, particularly with the ongoing success of clinical trials. For example, in April 2025, NeuroSense Therapeutics released positive results from its Phase 2b PARADIGM clinical trial for ALS treatment. The trial demonstrated that PrimeC, an experimental ALS treatment, has a notable effect on microRNA modulation, offering new hope for ALS patients by potentially slowing disease progression. Such breakthroughs highlight the growing focus on targeted and effective therapies for ALS. With increasing investment in research and the continued progress of clinical trials, including those focused on gene therapy and neuroprotection, the market is witnessing significant advancements that could revolutionize ALS treatment outcomes in the near future.
Amyotrophic Lateral Sclerosis Treatment Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global amyotrophic lateral sclerosis treatment market, along with forecasts at the global, regional, and country levels from 2025-2033. The market has been categorized based on ALS type, drug type, diagnosis type, treatment, and distribution channel.
Analysis by ALS Type:
- Sporadic ALS
- Familial ALS
Sporadic ALS stand as the largest component in 2024, holding around 68.5% of the market. The sporadic ALS segment dominates the amyotrophic lateral sclerosis (ALS) treatment market due to its higher prevalence compared to familial ALS. This widespread occurrence drives the demand for treatments, research, and healthcare resources dedicated to managing and slowing disease progression. Additionally, the lack of a clear genetic cause in sporadic ALS increases the complexity of treatment, leading to greater focus on developing targeted therapies. Consequently, the large patient population and the need for advanced therapies fuel the market growth in this segment.
Analysis by Drug Type:
- Marketed Drugs
- Emerging Drugs
Marketed drugs play a crucial role in the amyotrophic lateral sclerosis (ALS) treatment market by providing immediate solutions to manage the disease's progression. The market is currently driven by FDA-approved therapies such as Rilutek (Riluzole) and Radicava (Edaravone), which help slow ALS progression, extending survival and improving quality of life for patients. As awareness of ALS grows and the demand for effective treatments increases, the marketed drugs segment continues to expand. Enhanced regulatory support, as well as greater insurance coverage, has made these drugs more accessible to a broader patient base. This segment remains pivotal in addressing the unmet needs of ALS patients worldwide, while ongoing research supports the development of next-generation therapies.
Emerging drugs in the ALS treatment market are gaining increasing prominence as researchers focus on innovative therapeutic approaches to address ALS's complex pathology. The emergence of gene therapies, cell-based treatments, and novel oral drugs marks a turning point in ALS management. The development of drugs like PrimeC, currently in clinical trials, demonstrates the potential to modify disease progression through microRNA modulation. Growth drivers for this segment include rising investment in ALS-specific research, advancements in personalized medicine, and the success of early-stage trials. These emerging therapies offer hope for more effective treatments, potentially revolutionizing ALS care by targeting the underlying mechanisms of neurodegeneration.
Analysis by Diagnosis Type:
- Electromyogram
- MRI
- Blood and Urine Tests
- Spinal Tap
- Muscle Biopsy
Electromyogram (EMG) plays a crucial role in the diagnosis and management of amyotrophic lateral sclerosis (ALS). It is a primary tool for detecting electrical activity in muscles, allowing healthcare professionals to assess nerve damage, which is a hallmark of ALS. The growing adoption of EMG as a diagnostic tool drives market growth, as early detection is critical for effective disease management. Technological advancements in EMG devices, combined with an increasing number of ALS cases, contribute to its widespread use in clinical settings. Moreover, healthcare awareness and the growing focus on accurate diagnostic procedures further bolster the demand for EMG in ALS treatment.
Magnetic Resonance Imaging (MRI) has become an essential imaging technique for diagnosing and monitoring ALS. While MRI cannot definitively diagnose ALS, it is used to rule out other neurological conditions with similar symptoms, ensuring accurate diagnosis. The use of advanced MRI techniques, such as functional and diffusion tensor imaging, helps in tracking disease progression and monitoring treatment responses. As research into ALS treatments advances, MRI’s ability to offer insights into brain and spinal cord abnormalities continues to drive its significance in clinical practice. This growing role in monitoring ALS is spurring the demand for MRI equipment and services within the market.
Blood and urine tests are becoming increasingly important in the diagnosis and monitoring of ALS. These tests help in identifying biomarkers associated with ALS, aiding in early detection and improving disease management. As research into ALS biomarkers advances, blood and urine tests are evolving into essential tools for tracking disease progression and response to treatment. This evolution is driving demand for more advanced diagnostic methods, which is positively impacting the ALS treatment market. The increasing focus on personalized medicine and targeted therapies further emphasizes the importance of blood and urine tests in identifying suitable treatment options for ALS patients.
Analysis by Treatment:
- Medication
- Stem Cell Therapy
- Others
Medication leads the market with around 84.9% of market share in 2024. The medication segment dominates the amyotrophic lateral sclerosis (ALS) treatment market due to the critical role of pharmaceutical therapies in managing the disease. Currently, there are few effective treatment options, making medication essential for slowing disease progression and alleviating symptoms. Drug treatments like riluzole and edaravone are the only FDA-approved options, with ongoing research into new therapies targeting the underlying causes of ALS. As ALS is a progressive neurodegenerative disorder, medications are crucial in extending patients’ lifespan and improving quality of life. Furthermore, continued advancements in targeted treatments and clinical trials are further propelling the dominance of the medication segment in the market.
Analysis by Distribution Channel:
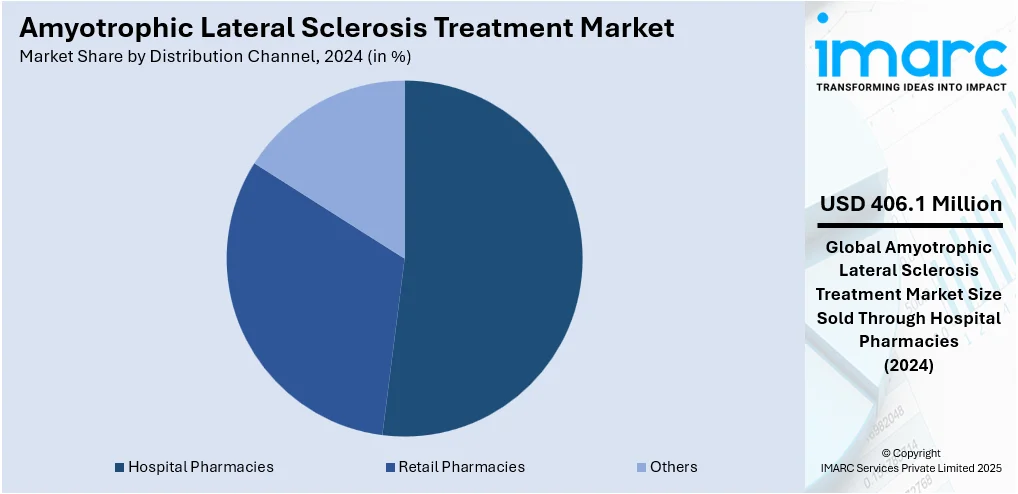
- Hospital Pharmacies
- Retail Pharmacies
- Others
Hospital pharmacies leads the market with around 51.6% of market share in 2024. The hospital pharmacies segment dominates the amyotrophic lateral sclerosis (ALS) treatment market due to their central role in the distribution of specialized medications. Hospitals are primary healthcare settings where ALS patients receive diagnosis, treatment, and continuous care. These pharmacies are equipped with access to advanced drugs, including experimental treatments and those under clinical trials, that are crucial for managing ALS. Furthermore, hospital pharmacies benefit from established relationships with pharmaceutical companies, allowing for faster and more reliable access to ALS therapies. Their ability to provide tailored prescriptions and comprehensive patient management services makes them a key player in the ALS treatment landscape.
Regional Analysis:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
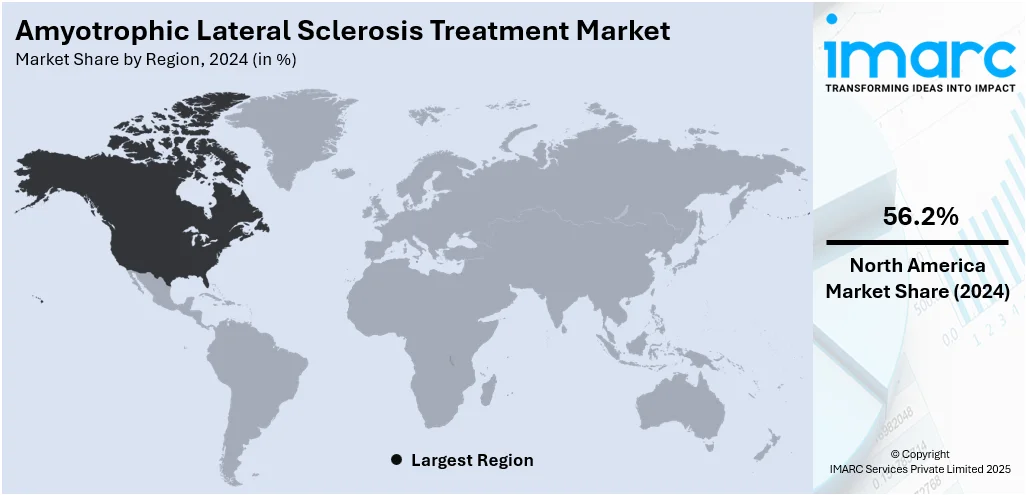
In 2024, North America accounted for the largest market share of over 56.2%. North America dominates the amyotrophic lateral sclerosis (ALS) treatment market due to several key factors, including robust healthcare infrastructure, advanced medical research, and high levels of disease awareness. For instance, in July 2025, Klotho Neurosciences' gene therapy candidate, KLTO‑202, received orphan drug status from the U.S. Food and Drug Administration (FDA) for the treatment of amyotrophic lateral sclerosis (ALS). This designation is aimed at supporting the development of treatments for rare diseases. KLTO‑202 is designed to deliver instructions for producing a protein that reduces inflammation and oxidative stress, which are critical factors in ALS progression. Preclinical studies have shown that the therapy helps protect nerve cells, improves muscle strength, and slows ALS progression. The company plans to launch a first-in-human trial by the third quarter of 2026, with hopes to further validate its potential as a new ALS treatment. The region benefits from strong support for clinical trials and innovation, with significant investment in ALS research from both public and private sectors. Additionally, comprehensive insurance coverage and well-established reimbursement policies make treatments more accessible to patients. The high incidence of ALS, combined with the growing geriatric population, further increases the demand for specialized care, contributing to North America’s dominance in the ALS treatment market.
Key Regional Takeaways:
United States Amyotrophic Lateral Sclerosis Treatment Market Analysis
In 2024, the United States held a market share of around 92% in North America. The United States amyotrophic lateral sclerosis (ALS) treatment market is primarily driven by scientific breakthroughs, regulatory support, and increasing patient advocacy. Advancements in understanding the molecular and genetic underpinnings of ALS, including discoveries related to SOD1 and C9orf72 mutations, are catalyzing the development of targeted therapies such as antisense oligonucleotides, gene therapies, and small-molecule disease modifiers. Regulatory agencies are also streamlining fast-track and orphan drug pathways, which has swiftly translated promising preclinical findings into clinical trials and accelerated approval timelines. For instance, in May 2025, the U.S. Food and Drug Administration (FDA) permitted New York-based BrainStorm Cell Therapeutics Inc. to start the Phase 3b clinical study of its NurOwn (autologous MSC-NTF cells) for the treatment of amyotrophic lateral sclerosis (ALS). The FDA previously approved the trial design through a Special Protocol Assessment (SPA), ensuring that the study's endpoints and statistical techniques are suitable for the eventual submission of a Biologics License Application (BLA). With this approval, the company can now start enrolling patients for this novel treatment. Additionally, breakthroughs in biomarkers, ranging from imaging modalities to neurofilament proteins, are enabling more precise patient stratification, earlier diagnosis, and improved evaluation of treatment efficacy. Pharmaceutical and biotech investment is also increasing, with numerous collaborations and licensing deals fostering a robust pipeline.
Asia Pacific Amyotrophic Lateral Sclerosis Treatment Market Analysis
The Asia Pacific amyotrophic lateral sclerosis (ALS) treatment market is expanding due to scientific advancements, growing healthcare infrastructure, and rising patient awareness. Pharmaceutical and biotech firms are expanding clinical trial operations in the region, leveraging increasingly capable research centers in countries such as Japan, South Korea, China, and Australia to gain access to diverse genetic populations and expedite recruitment. Moreover, public and private sector investments to support innovation in neurodegenerative disease therapies are steadily increasing, creating a favorable environment for the development of new ALS treatment therapies. For instance, in May 2025, Ahmedabad-based Zydus Group successfully secured a Fast Track designation from the U.S. Food and Drug Administration (FDA) for its new neuroinflammatory drug, Usnoflast, for the treatment of amyotrophic lateral sclerosis (ALS). This Fast Track designation highlights the growing need for the development of effective treatment therapies for ALS in India. Expanding diagnostic networks and specialist neurology clinics are also facilitating earlier disease recognition and timely intervention, thereby supporting the adoption of ALS treatment therapies.
Europe Amyotrophic Lateral Sclerosis Treatment Market Analysis
The growth of the Europe amyotrophic lateral sclerosis (ALS) treatment market is largely fueled by medical innovations, regulatory alignment, a large number of geriatric population and patient empowerment. In 2024, the EU population was estimated at 449.3 million people and more than one-fifth (21.6%) of it was aged 65 years and over. Scientific advancements in gene editing and RNA therapeutics, particularly antisense oligonucleotides targeting common ALS-associated mutations, are forming the backbone of a growing clinical pipeline, supported by international funding and research consortia. Harmonized regulatory frameworks across the EU, including adaptive approval schemes and orphan designation incentives, are also expediting market entry for breakthrough therapies. Additionally, patient advocacy groups and European ALS registries are playing a central role in clinical trial recruitment and the collection of real-world data. Health technology assessment bodies are increasingly adopting value-based pricing models that consider long-term disease-modifying effects, improving reimbursement prospects for high-cost treatments. Collaborative partnerships between academic institutions, biotech firms, and pharmaceutical companies are fueling translational research, enabling the production and commercialization of novel treatment therapies.
Latin America Amyotrophic Lateral Sclerosis Treatment Market Analysis
The Latin America amyotrophic lateral sclerosis (ALS) treatment market is experiencing robust growth due to increasing healthcare digitization and increasing awareness among the people in the country. In Brazil, an average of 12,000 patients are diagnosed with ALS each year. As remote care becomes more widely adopted, patients in underserved and rural areas are gaining better access to specialized neurological consultations and ongoing disease management. Public-private collaborations are also supporting investment in rare disease frameworks, creating opportunities for greater funding and awareness. Besides this, efforts to harmonize regulatory standards across countries are streamlining the approval process for innovative treatments, further encouraging market entry.
Middle East and Africa Amyotrophic Lateral Sclerosis Treatment Market Analysis
The Middle East and Africa Amyotrophic Lateral Sclerosis (ALS) treatment market is experiencing gradual growth, driven by increasing awareness, improving diagnostic capabilities, and a rise in healthcare investments across the region. As reported, in terms of GDP, the aggregate healthcare expenditure in the KSA increased from 4.4% of GDP in 2001 to 5.97% of GDP in 2021. Although ALS remains a relatively rare neurodegenerative disorder, early detection and the availability of treatment options such as riluzole, edaravone, and supportive therapies are expanding. Countries like the UAE and Saudi Arabia are witnessing enhanced access to specialized neurology care, while collaborations with global pharmaceutical companies are supporting the introduction of advanced treatment options.
Competitive Landscape:
The competitive landscape of the amyotrophic lateral sclerosis treatment market is dynamic, with numerous players focused on developing innovative therapies to address unmet needs. Companies are heavily investing in research and development to create new drug classes, particularly targeting neuroprotection and disease-modifying treatments. For instance, in November 2024, Eisai Co., Ltd. launched Rozebalamin® for Injection 25 mg (mecobalamin) in Japan for the treatment of amyotrophic lateral sclerosis (ALS), marking a significant milestone in ALS treatment. This drug aims to slow the progression of functional impairment in ALS patients. The approval is based on the successful results of the JETALS Phase III clinical trial, which demonstrated the drug’s effectiveness compared to a placebo. Strategic collaborations between pharmaceutical companies, academic institutions, and research organizations are accelerating progress in clinical trials and bringing new treatments to market faster. The growing focus on personalized medicine and biomarkers for early detection further drives competition. As a result, the amyotrophic lateral sclerosis treatment market forecast predicts continued innovation and a rise in the number of treatment options, increasing market competition and expanding opportunities for growth in the coming years.
The report provides a comprehensive analysis of the competitive landscape in the amyotrophic lateral sclerosis treatment market with detailed profiles of all major companies, including:
- AB Science
- Ascend Pharmaceuticals LLC
- BrainStorm Cell Therapeutics Inc.
- Corestem Inc.
- Cytokinetics Inc.
- Eledon Pharmaceuticals Inc.
- Ionis Pharmaceuticals Inc.
- ITF Pharma (Italfarmaco S.p.A)
- Mitsubishi Chemical Group Corpotion
- Revalesio Corporation
- Treeway B.V.
Latest News and Developments:
- June 2025: Amylyx Pharmaceuticals, Inc. reported that AMX0114, an experimental antisense oligonucleotide (ASO) that targets calpain-2 for the treatment of individuals with amyotrophic lateral sclerosis (ALS), was awarded Fast Track designation by the U.S. Food and Drug Administration (FDA). This marks a significant milestone in the company's goal to create potential treatment therapies for ALS patients.
- June 2025: Spinogenix, Inc. reported that its SPG302 has been designated as an orphan drug (ODD) by the European Medicines Agency (EMA) for the treatment of individuals with Amyotrophic Lateral Sclerosis (ALS). SPG302 is under development as a pioneering synaptic regeneration therapy for ALS that has the potential to improve breathing, mobility, and cognitive function for individuals suffering from ALS.
- April 2025: Ionis Pharmaceuticals, Inc. reported that the U.S. Food and Drug Administration (FDA) approved QALSODY (tofersen) 100 mg/15 mL injection, a product of its partner Biogen, for the treatment of Amyotrophic Lateral Sclerosis (ALS) in adults with a mutation in the superoxide dismutase 1 (SOD1) gene. The application was granted fast approval as patients receiving QALSODY exhibited a decrease in plasma neurofilament light chain (NfL).
- April 2025: MedicNova, Inc. reported that the NIH-funded Expanded Access Program (EAP) experiment to assess MN-166 (ibudilast) in patients with Amyotrophic Lateral Sclerosis (ALS) successfully enrolled its first patient. This important milestone marks the commencement of a crucial trial that will give ALS patients ineligible for the ongoing Phase 2/3 COMBAT-ALS trial access to MN-166.
- April 2025: Sineugene Therapeutics announced that the U.S. Food and Drug Administration (FDA) approved a Phase 1/2a trial of its experimental gene treatment, SNUG01, in patients with amyotrophic lateral sclerosis (ALS). The international study will use a dose escalation and expansion procedure to evaluate the treatment's safety, tolerance, and initial effectiveness in adults with ALS.
Amyotrophic Lateral Sclerosis Treatment Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| ALS Types Covered | Sporadic ALS, Familial ALS |
| Drug Types Covered | Marketed Drugs, Emerging Drugs |
| Diagnosis Types Covered | Electromyogram, MRI, Blood and Urine Tests, Spinal Tap, Muscle Biopsy |
| Treatments Covered | Medication, Stem Cell Therapy, Others |
| Distribution Channels Covered | Hospital Pharmacies, Retail Pharmacies, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | AB Science, Ascend Pharmaceuticals LLC, BrainStorm Cell Therapeutics Inc., Corestem Inc., Cytokinetics Inc., Eledon Pharmaceuticals Inc., Ionis Pharmaceuticals Inc., ITF Pharma (Italfarmaco S.p.A), Mitsubishi Chemical Group Corpotion, Revalesio Corporation and Treeway B.V. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the amyotrophic lateral sclerosis treatment market from 2019-2033.
- The amyotrophic lateral sclerosis treatment market research report provides the latest information on the market drivers, challenges, and opportunities in the global market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the amyotrophic lateral sclerosis treatment industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
The amyotrophic lateral sclerosis (ALS) treatment market was valued at USD 787.02 Million in 2024.
The amyotrophic lateral sclerosis (ALS) treatment market is projected to exhibit a CAGR of 4.15% during 2025-2033, reaching a value of USD 1,134.53 Million by 2033.
The market is driven by the rising prevalence of ALS, increasing awareness about neurodegenerative disorders, and significant advancements in drug development and clinical trials. Continued research into gene therapy, stem cell therapy, and novel drug mechanisms is contributing to market growth. In addition, growing investment from both public and private sectors, favorable regulatory support, and increasing availability of orphan drug designations are further accelerating the development of ALS treatment options worldwide.
North America currently dominates the amyotrophic lateral sclerosis (ALS) treatment market, accounting for a share of over 56.2% in 2024. The region is characterized by strong healthcare infrastructure, advanced research facilities, and the presence of key pharmaceutical companies actively working on ALS therapeutics. Increased awareness, access to experimental treatments, and supportive reimbursement frameworks further strengthen North America's leadership in the global ALS treatment market.
Some of the major players in the amyotrophic lateral sclerosis (ALS) treatment market include AB Science, Ascend Pharmaceuticals LLC, BrainStorm Cell Therapeutics Inc., Corestem Inc., Cytokinetics Inc., Eledon Pharmaceuticals Inc., Ionis Pharmaceuticals Inc., ITF Pharma (Italfarmaco S.p.A), Mitsubishi Chemical Group Corpotion, Revalesio Corporation, Treeway B.V., etc.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












