
Digital Dose Inhaler Market Size, Share, Trends and Forecast by Type, Product, and Region, 2025-2033
Digital Dose Inhaler Market Size and Share:
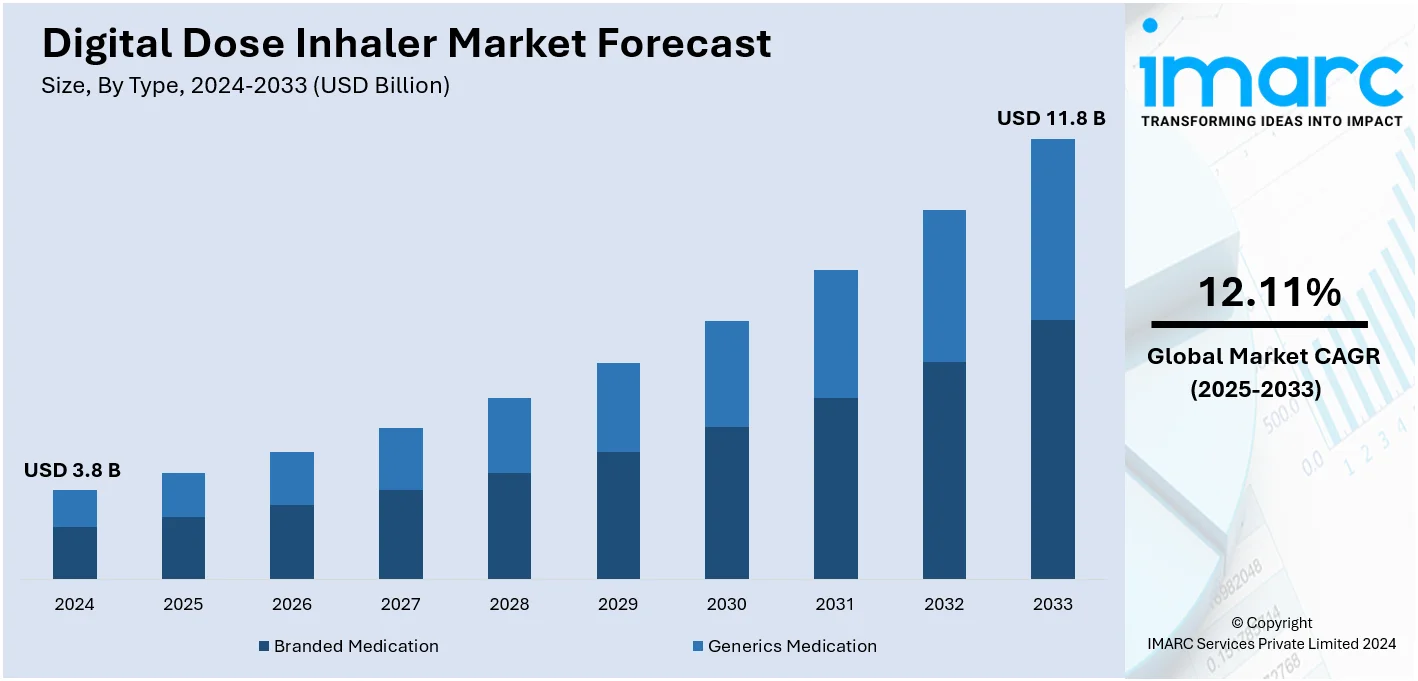
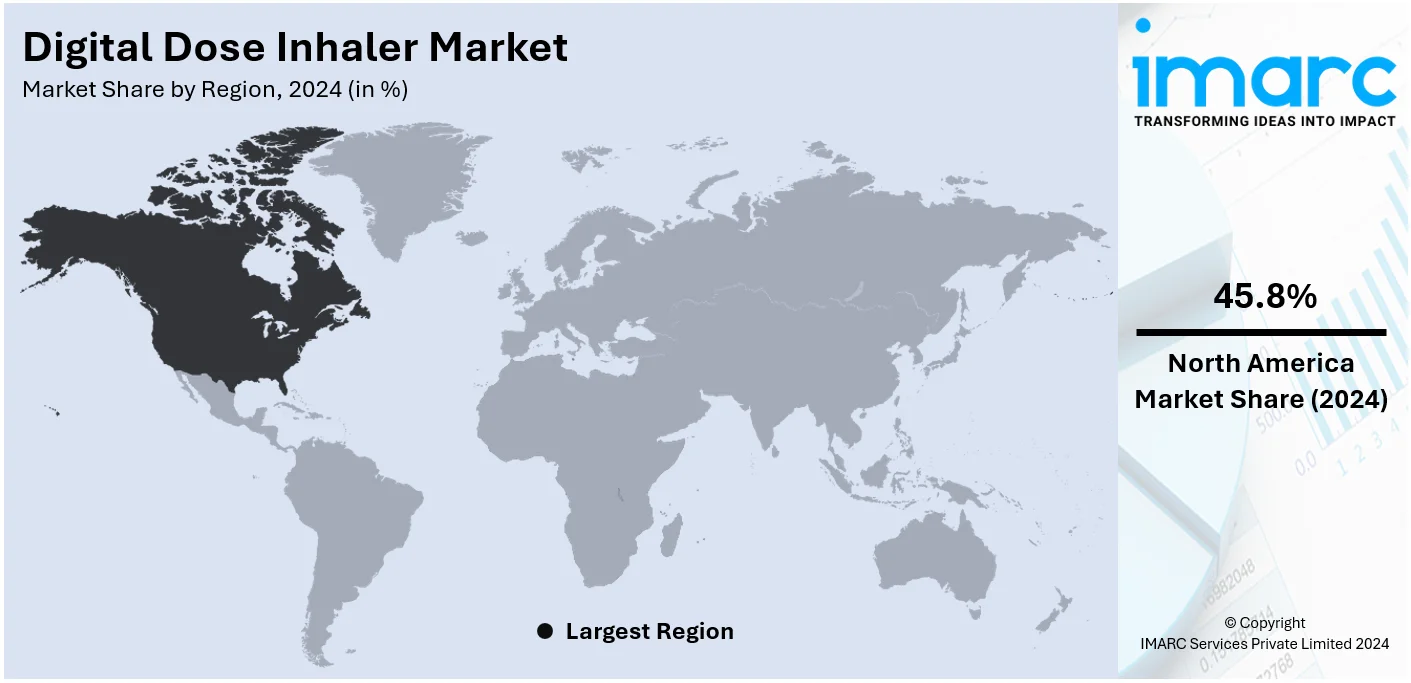
The global digital dose inhaler market size was valued at USD 3.8 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 11.8 Billion by 2033, exhibiting a CAGR of 12.11% from 2025-2033. North America currently dominates the market, holding a market share of over 45.8% in 2024. This is due to the increasing focus on the management of chronic respiratory disease, technological advancements that allow real-time adjustments, and the increasing prevalence of chronic respiratory diseases, including chronic obstructive pulmonary disease (COPD) and asthma.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 3.8 Billion |
|
Market Forecast in 2033
|
USD 11.8 Billion |
| Market Growth Rate (2025-2033) | 12.11% |
The global digital dose inhaler market is largely driven by several key factors that include health technology advancement, the growth in the number of cases of respiratory disorders, such as asthma and COPD, and digitalized dose inhalers offering such enhanced features as dose tracking, reminders, and real-time feedback, thereby leading to improvements in the outcome of a patient's treatment. The other major driver is the growing awareness about the benefits of connected healthcare devices among patients and healthcare providers. These devices enable remote monitoring and data sharing, which leads to better management of chronic conditions. The growing elderly population, which is more prone to respiratory ailments, also increases demand. Supportive government policies and initiatives promoting digital health solutions also play a pivotal role. Also, artificial intelligence and mobile applications being integrated into inhaler technology attract the attention of tech-savvy consumers, thus fueling market growth.
The United States emerged as a key regional market for the digital dose inhaler. There is a huge prevalence of respiratory disorders, which includes conditions such as asthma and COPD, affecting millions each year in the U.S. Also, the high health infrastructure in the country coupled with the widespread acceptance of the latest medical appliances, has increased the acceptance of digital dose inhalers, and awareness about adhering to prescription-based medication therapy further pushed the demand in the country. Initiatives undertaken by the government toward digital health technology and the proper reimbursement policy made digital inhalers easily introduced into routine care. With the rise of telemedicine and remote monitoring systems, the importance of digital inhalers is being multiplied. The need for patient-centric solutions and an elderly population also drives the market of U.S.
Digital Dose Inhaler Market Trends:
Increasing prevalence of chronic respiratory diseases
The increased incidence rate of chronic respiratory diseases amongst the masses globally due to diseases such as asthma and COPD is propelling growth in the market. An article published by PMC claims, "One in three adults worldwide has multiple chronic conditions.". Further, people suffering from respiratory disorders who need them to manage those conditions demand an enhanced drug delivery system. Except for the above reasons, DDIs provide a state-of-the-art solution as the medication adheres accurately, and at the same time, provides feedback for patients as well as to their doctors. Which presents an upbeat view of the market. By this, the information gathered may prove useful in tailoring treatment plans, enhancing adherence to medication regimens, and perhaps reducing hospitalizations due to CRDs.
Technological advancements for real time adjustments
To enhance patient care and satisfaction, companies are continually upgrading their DDIs. These devices offer features such as dose counting, adherence monitoring, and data transmission to healthcare providers. This goes with these features to encourage better management of disease, allow the real-time alteration of treatment plans, and assure that the patients are appropriately adhering to their schedules for medication, which shows a positive market outlook. The global chronic disease management market size reached USD 6.5 Billion in 2024. In addition, technology in DDIs helps the patients get instant feedback about their inhalation technique so that the treatment is perfectly conducted. Thus, through such innovations, the severity of respiratory diseases is taken care of, and the care given to patients with respiratory disorders is greatly improved.
Increasing emphasis on managing chronic respiratory diseases
The growing concern among patients and healthcare providers over the management of chronic respiratory disease (CRD) is further propelling the demand in the market. Further, there is more demand for better management strategies of CRDs. As per PubMed Central, chronic respiratory conditions have a prevalence of around 545 million people, which amounts to 7.4 percent of the global population. In addition, DDIs can measure medication adherence and provide actionable feedback, that works at the heart of broader chronic respiratory disease management strategies. Additionally, they ensure a more comprehensive understanding of adherence to treatment and disease for the healthcare providers, who may then intervene in real time and improve the conditions and results for the patients better. Moreover, the surging adoption of DDIs as they help in facilitating improved self-management is a driver for market advancement.
Digital Dose Inhaler Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global digital dose inhaler market, along with forecasts at the global, regional, and country levels from 2025-2033. The market has been categorized based on type and product.
Analysis by Type:
- Branded Medication
- Generics Medication
Branded medication is the leading market share holder in the market due to its established trust among healthcare providers and patients. These medications are often associated with consistent quality, rigorous clinical trials, and superior efficacy, making them a preferred choice for treating chronic respiratory conditions like asthma and COPD. Pharmaceutical companies' strong promotional strategies and ongoing innovation in branded formulations further drive their market share. Further, the availability of branded digital dose inhalers with high technology increases patient compliance and hence fuels the growth of the market. In the developed regions, such as North America, favorable reimbursement policies and insurance coverage for branded drugs also contribute to their strong market position. The competition in the market by generics will not affect the demand for branded drugs due to their reliability and technological integration.
Analysis by Product:
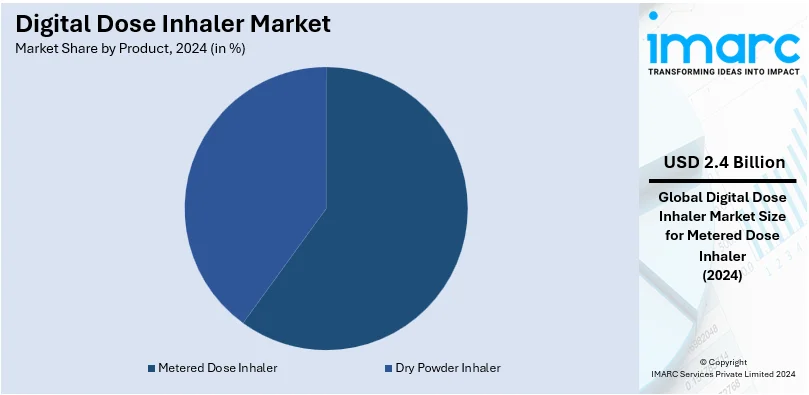
- Metered Dose Inhaler
- Dry Powder Inhaler
The metered dose inhalers hold a 64.4% market share in 2024. It is due to their ease of use, portability, and efficiency in providing a measured dose of medication straight into the lungs. MDIs are more recommended for asthma and COPD, especially for acute presentations requiring rapid relief. Technological improvements-including the addition of features that enable digital tracking, in MDIs, are contributing toward adherence and attractiveness to patients as well as healthcare providers. Moreover, the wide availability of MDIs in branded and generic formulations will ensure access across multiple socio-economic segments. Supporting the cause are also positive policies for healthcare and distribution infrastructures in developed regions like North America. The rising incidence of respiratory disease worldwide accelerates MDI market growth further and creates a cornerstone of inhalation therapy.
Regional Analysis:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
The North American region is the largest market with a share of 45.8%, due to the high penetration of advanced respiratory devices. This region has an already developed healthcare infrastructure, which easily accommodates new technologies such as digital dose inhalers and connected respiratory devices. Top pharmaceutical and medical device companies operating in this region add further strength to the market through continuous innovative pipelines and robust distribution networks. In addition, the growing focus on enhancing respiratory care solutions, through government policies and increased healthcare expenditure, further contributes to market growth. The increasing awareness about the significance of the early diagnosis and effective management of respiratory conditions leads to enhanced adoption rates both by the patients and healthcare providers. Rising respiratory disorders, which include asthma, COPD, and other chronic diseases, will push the demand in North America, which leads the world in market shares.
Key Regional Takeaways:
United States Digital Dose Inhaler Market Analysis
The United States hold 89.40% of the digital dose inhaler market share in North America. The rising prevalence of chronic diseases, including respiratory disorders such as asthma and COPD, drives the digital dose inhaler market in the United States. According to the United States Department of Health and Human Services, 129 million Americans live with at least one major chronic condition. This indicates the growing requirement for modern solutions to manage long-term health issues effectively. Digital dose inhalers, with their integration of mobile apps and real-time medication tracking features, are gaining momentum with their role in improving patient adherence and outcomes. Technological advancements, coupled with widespread adoption of connected healthcare infrastructure, further fuel market growth. Government support through favorable reimbursement policies and initiatives promoting digital health technologies adds momentum. Moreover, the post-COVID-19 increase in telemedicine and remote monitoring tools has underlined the need for smart inhalers in chronic disease management. The presence of key market players focusing on research and development ensures a continuous influx of innovative, patient-centric solutions, making digital dose inhalers an essential component in the evolving U.S. healthcare landscape.
Europe Digital Dose Inhaler Market Analysis
The digital dose inhaler market in Europe is led by the region's emphasis on healthcare innovation and chronic disease management. An aging population significantly contributes to market growth, as older individuals are more susceptible to respiratory conditions like asthma and COPD. As of January 1, 2023, Eurostat estimated that the EU population was approximately 448.8 million, with over one-fifth (21.3%) aged 65 years and above. This demographic shift underlines the increasing demand for advanced healthcare solutions tailored to the elderly. Europe's strict regulatory environment promotes safe and efficient digital health products, and research and innovation funding push the boundaries. The increased value-based healthcare systems that are patient adherence-centered align with the characteristics of digital dose inhalers, and partnerships between healthcare providers and technology companies are helping develop connected ecosystems for better management of disease. The region’s increasing emphasis on sustainability has also encouraged the adoption of eco-friendly inhaler designs. With strong infrastructure and patient-focused strategies, Europe remains a key market for digital dose inhaler innovations.
Asia Pacific Digital Dose Inhaler Market Analysis
The Asia Pacific market of digital dose inhalers is booming as people are becoming urban, leading to higher levels of respiratory infections like bronchitis and asthma. As of 2021, census data of China indicates that 64% of people are living in cities. India has its records showing that 37% of India is staying within cities. High rates of urbanization, associated with growing air pollution within denser and more populated cities, significantly contribute to respiratory disorders, as these populations necessitate improved healthcare solutions. With increasing middle-class consumers that hold higher disposable income levels, there is an expansion of the adoption of smart healthcare devices. Several Indian and Chinese governments, by making huge investments in infrastructural building health care, are offering and supporting digital health services across this region. The wide acceptance of smartphones and digital media has led to the integration of smart inhalers with mobile applications that can track real-time usage, hence enhancing the patient's adherence and managing the disease better. Further, awareness campaigns have increased the penetration of the market.
Latin America Digital Dose Inhaler Market Analysis
The digital dose inhaler market in Latin America is driven by the rising prevalence of chronic diseases and increasing demand for advanced healthcare solutions. For instance, according to PubMed Central, Brazil alone registers an estimated 928,000 deaths annually due to chronic diseases, which calls for better management tools. Urbanization and air pollution increase respiratory conditions, thereby boosting the demand for smart inhalers. Government initiatives to promote digital health solutions and the growing accessibility of smartphones facilitate the adoption of connected inhalers, enabling improved patient adherence and real-time monitoring, thus driving digital dose inhaler market growth in the region.
Middle East and Africa Digital Dose Inhaler Market Analysis
The digital dose inhaler market in the Middle East is driven by rising respiratory disorders due to factors like air pollution and desert dust. Governments are prioritizing healthcare investments to modernize infrastructure and adopt advanced medical technologies. For instance, the UAE, as part of its 2022-2026 fiscal plan, allocated USD 1.56 Billion (AED 5.74 Billion) to healthcare and community prevention services, marking a 10.4% increase from its 2024 budget. This commitment, coupled with the increasing adoption of digital health and improved internet access, supports the increased use of smart inhalers for better disease management and patient adherence in the region.
Competitive Landscape:
The digital dose inhaler market players are working on innovation and strategic collaboration to enhance their market position. Companies are designing high-tech inhalers with sensors and digital tracking systems that can track the use of medication and provide real-time feedback to patients and healthcare providers. These devices are Bluetooth and mobile app enabled, thus making adherence to treatment plans easier. Smart inhalers with more advanced features are also being developed due to partnerships with healthcare organizations and tech firms. Furthermore, market players are expanding their geographical reach through mergers, acquisitions, and distribution agreements to tap into emerging markets. Investments in research and development to improve inhaler efficiency and user experience further highlight the competitive dynamics of this growing market.
The report provides a comprehensive analysis of the competitive landscape in the digital dose inhaler market with detailed profiles of all major companies, including:
- 3M Company
- AstraZeneca plc
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Limited
- H&T Presspart Manufacturing Ltd.
- Koninklijke Philips N.V.
- Lupin Limited
- Novartis AG
- OPKO Health Inc.
- Propeller Health (ResMed)
- Sensirion AG Switzerland
- Teva Pharmaceutical Industries Ltd.
(Please note that this is only a partial list of the key players, and the complete list is provided in the report.)
Latest News and Developments:
- October 2024: Modivcare partnered with Tenovi to deliver Adherium's Hailie® Smart Inhalers, aiming to enhance care for patients with chronic respiratory diseases. The collaboration combines Tenovi’s remote monitoring technology and Modivcare’s condition management services to improve medication adherence, reduce hospitalizations, and lower healthcare costs.
- May 2024: Leicester launched an NHS trial of smart inhalers for children with asthma. The trial, involving 300 children, uses Hailie digital inhalers to monitor medication use and provide feedback via a smartphone app. The study aims to improve asthma control and reduce hospital visits, with results expected in early 2025.
- September 2024: AstraZeneca has completed studies for Breztri/Trixeo Aerosphere (budesonide/glycopyrronium/formoterol fumarate) to transition to a next-generation propellant with 99.9% lower global warming potential. This makes Breztri the first digital dose inhaler in AstraZeneca's portfolio to use the new propellant. The company is also working on transitioning to other inhalers to reduce their carbon footprint, contributing less than 0.04% to global greenhouse gas emissions.
- June 2024: Aseptika launched the PUFFClicker3, a inhaler dose tracker that is compatible with pMDI and DPI. It supports 101 inhalers and uses mobile IoT technology to transmit real-time data to improve adherence in the treatment of respiratory diseases for patients. The device offers multiple languages and does not need a smartphone to aid carers in monitoring usage.
- March 2024: BerryHaler, a dual-chamber dry powder inhaler with a dose counter released by the Berry Global. The product is designed for combination drug delivery in COPD, asthma, and lung disorders. The device can deliver up to 30 doses of double or triple formulations simultaneously. It has a bi-directional mouthpiece cover, audible signals for proper use, and a dose counter to help patients track medication usage. The BerryHaler is designed for ease of use across various patient profiles, including seniors and children.
Digital Dose Inhaler Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Types Covered | Branded Medication, Generics Medication |
| Products Covered | Metered Dose Inhaler, Dry Powder Inhaler |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | 3M Company, AstraZeneca plc, GlaxoSmithKline plc, Glenmark Pharmaceuticals Limited, H&T Presspart Manufacturing Ltd., Koninklijke Philips N.V., Lupin Limited, Novartis AG, OPKO Health Inc., Propeller Health (ResMed), Sensirion AG Switzerland, Teva Pharmaceutical Industries Ltd., etc. (Please note that this is only a partial list of the key players, and the complete list is provided in the report.) |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the digital dose inhaler market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the global digital dose inhaler market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the digital dose inhaler industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
A digital dose inhaler combines the traditional functionality of an inhaler with smart technology to track medication use, send reminders, and provide immediate feedback on inhalation techniques. It connects to apps or devices to help improve adherence, optimize treatment, and share data with healthcare providers for better respiratory disease management.
The digital dose inhaler market was valued at USD 3.8 Billion in 2024.
IMARC estimates the global digital dose inhaler market to exhibit a CAGR of 12.11% during 2025-2033.
The major drivers for the market are the increasing focus on the management of chronic respiratory disease, technological advancements that allow real-time adjustments, and the increasing prevalence of chronic respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD).
In 2024, branded medication represented the largest segment due to its established trust among healthcare providers and patients.
Metered dose inhaler leads the market due to their ease of use, portability, and efficiency in providing a measured dose of medication straight into the lungs.
On a regional level, the market has been classified into North America, Asia Pacific, Europe, Latin America, and Middle East and Africa, wherein North America currently dominates the global market.
Some of the major players in the global digital dose inhaler market include 3M Company, AstraZeneca plc, GlaxoSmithKline plc, Glenmark Pharmaceuticals Limited, H&T Presspart Manufacturing Ltd., Koninklijke Philips N.V., Lupin Limited, Novartis AG, OPKO Health Inc., Propeller Health (ResMed), Sensirion AG Switzerland, Teva Pharmaceutical Industries Ltd., etc.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












