
Japan Medical Cannabis Market Size, Share, Trends and Forecast by Species, Derivatives, Application Route of Administration, End Use, and Region, 2026-2034
Japan Medical Cannabis Market Overview:
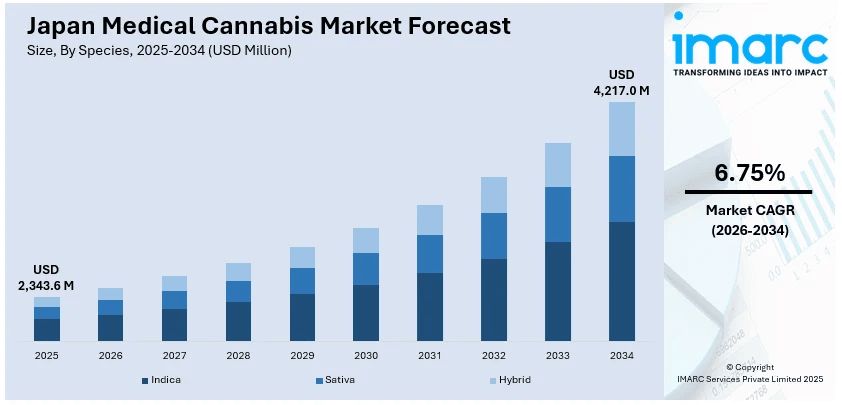
The Japan medical cannabis market size reached USD 2,343.6 Million in 2025. Looking forward, IMARC Group expects the market to reach USD 4,217.0 Million by 2034, exhibiting a growth rate (CAGR) of 6.75% during 2026-2034. The market is experiencing significant growth, driven by regulatory reforms, rising CBD acceptance, and growing clinical research. Moreover, legal changes have also opened pathways for cannabis-derived pharmaceuticals, signaling cautious but steady progress in therapeutic cannabis adoption.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 2,343.6 Million |
| Market Forecast in 2034 | USD 4,217.0 Million |
| Market Growth Rate 2026-2034 | 6.75% |
Japan Medical Cannabis Market Trends:
Growing Interest in CBD-Based Products
The popularity of CBD-based products is rising steadily in Japan, especially in the wellness and therapeutic segments. The Japanese medical cannabis market growth is being largely fueled by consumer interest in non-psychoactive cannabidiol (CBD) for relief from stress, chronic pain, inflammation, and sleep-related issues. THC-free CBD oils, capsules, creams, and beverages are increasingly available through health stores, e-commerce platforms, and specialty retailers. For instance, in May 2024, Sunderstorm announced its plans to launch KANHA in Japan, introducing two product lines, KRx by KANHA and KANHA Wellness, targeting medical and wellness consumers. This marks KANHA's entry into its second Asian market, following Thailand. The products feature ultra-clean, no-THC CBD oil and are backed by strategic partnerships with key industry players. This growing demand is also supported by Japan’s aging population, which is more open to alternative therapies for conditions like arthritis, insomnia, and anxiety. While THC remains strictly regulated, the legal acceptance of CBD with 0% THC has created a niche yet rapidly expanding market. International and domestic brands are exploring this space with a focus on safety, transparency, and efficacy. These developments contribute positively to the Japan medical cannabis market outlook over the coming years.
To get more information on this market Request Sample
Rising Pharmaceutical Research and Clinical Trials
Japan is gradually opening the door to medical cannabis through a growing emphasis on pharmaceutical research and clinical trials. This shift is being supported by recent legal changes aimed at enabling controlled medical applications of cannabis. For instance, in December 2024, Japan government amended its Cannabis Control Law, permitting the use of cannabis-derived drugs, particularly cannabidiol (CBD). The law allows for the development of CBD pharmaceuticals and clinical trials while maintaining strict penalties for the self-use of cannabis products deemed narcotics under the Narcotics and Psychotropic Substances Control Law. Institutions and biotech firms are conducting studies on cannabinoids, particularly CBD and synthetic THC, to evaluate their therapeutic potential for conditions such as treatment-resistant epilepsy, cancer-related pain, multiple sclerosis, and inflammatory disorders. These trials are being conducted under strict government oversight, reflecting Japan’s cautious yet progressive approach to cannabis-based medicine. Results from global studies are also influencing domestic research agendas and public policy discussions. The Ministry of Health, Labour and Welfare has shown interest in facilitating data-driven pathways for limited medical use, which may eventually lead to regulatory approval. The medical community’s increasing involvement signals a shift in perception, moving from stigma to scientific inquiry. This expanding research landscape is expected to play a key role in shaping the Japan medical cannabis market share.
Japan Medical Cannabis Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the market, along with forecasts at the regional level for 2026-2034. Our report has categorized the market based on species, derivatives, application route of administration, and end use.
Species Insights:
- Indica
- Sativa
- Hybrid
The report has provided a detailed breakup and analysis of the market based on the species. This includes indica, sativa, and hybrid.
Derivatives Insights:
- Cannabidiol (CBD)
- Tetrahydrocannabinol (THC)
- Others
A detailed breakup and analysis of the market based on the derivatives have also been provided in the report. This includes cannabidiol (CBD), tetrahydrocannabinol (THC), and others.
Application Insights:
Access the comprehensive market breakdown Request Sample
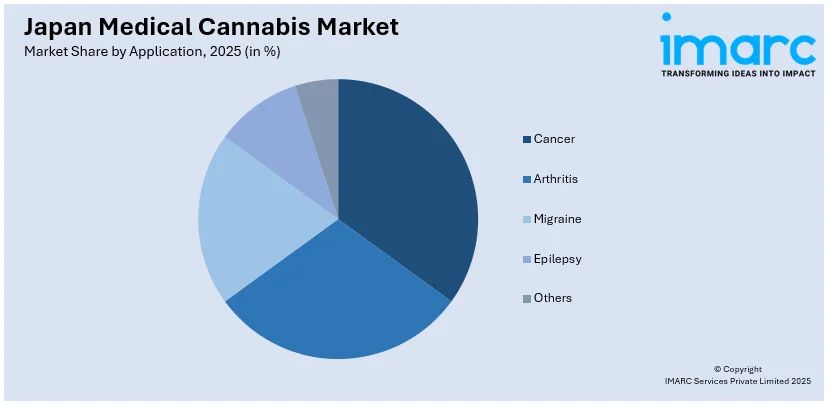
- Cancer
- Arthritis
- Migraine
- Epilepsy
- Others
A detailed breakup and analysis of the market based on the application have also been provided in the report. This includes cancer, arthritis, migraine, epilepsy, and others.
Route of Administration Insights:
- Oral Solutions and Capsules
- Vaporizers
- Topicals
- Others
A detailed breakup and analysis of the market based on the route of administration have also been provided in the report. This includes oral solutions and capsules, vaporizers, topicals, and others.
End Use Insights:
- Pharmaceutical Industry
- Research and Development Centres
- Others
A detailed breakup and analysis of the market based on the end use have also been provided in the report. This includes the pharmaceutical industry, research and development centers, and others.
Regional Insights:
- Kanto Region
- Kansai/Kinki Region
- Central/Chubu Region
- Kyushu-Okinawa Region
- Tohoku Region
- Chugoku Region
- Hokkaido Region
- Shikoku Region
The report has also provided a comprehensive analysis of all the major regional markets, which include Kanto Region, Kansai/Kinki Region, Central/Chubu Region, Kyushu-Okinawa Region, Tohoku Region, Chugoku Region, Hokkaido Region, and Shikoku Region.
Competitive Landscape:
The market research report has also provided a comprehensive analysis of the competitive landscape. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
Japan Medical Cannabis Market News:
- In December 2024, AstraSana Japan Co., Ltd. announced its partnership with GSI Creos Corporation, marking a significant investment in Japan’s CBD industry. This alliance aims to enhance the wholesale distribution of high-quality Swiss-made CBD products across Japan, emphasizing safety, reliability, and customer satisfaction while addressing market challenges and promoting growth in the sector.
- In July 2023, CBDTokyo announced the launch of its highest concentration CBD oil, “CBD Oil 18.2% (IPPATSU),” featuring 1820mg of CBD in a 10ml bottle. Designed for users seeking accessible self-care, it supports relaxation and sleep.
Japan Medical Cannabis Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Species Covered | Indica, Sativa, Hybrid |
| Derivatives Covered | Cannabidiol (CBD), Tetrahydrocannabinol (THC), Others |
| Applications Covered | Cancer, Arthritis, Migraine, Epilepsy, Others |
| Route of Administrations Covered | Oral Solutions and Capsules, Vaporizers, Topicals, Others |
| End Uses Covered | Pharmaceutical Industry, Research and Development Centres, Others |
| Regions Covered | Kanto Region, Kansai/Kinki Region, Central/Chubu Region, Kyushu-Okinawa Region, Tohoku Region, Chugoku Region, Hokkaido Region, Shikoku Region |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the Japan medical cannabis market performed so far and how will it perform in the coming years?
- What is the breakup of the Japan medical cannabis market on the basis of species?
- What is the breakup of the Japan medical cannabis market on the basis of derivatives?
- What is the breakup of the Japan medical cannabis market on the basis of application?
- What is the breakup of the Japan medical cannabis market on the basis of route of administration?
- What is the breakup of the Japan medical cannabis market on the basis of end use?
- What is the breakup of the Japan medical cannabis market on the basis of region?
- What are the various stages in the value chain of the Japan medical cannabis market?
- What are the key driving factors and challenges in the Japan medical cannabis market?
- What is the structure of the Japan medical cannabis market and who are the key players?
- What is the degree of competition in the Japan medical cannabis market?
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Japan medical cannabis market from 2020-2034.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Japan medical cannabis market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Japan medical cannabis industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












