Active Pharmaceutical Ingredients Cost Model: Breaking Down the Economics of Drug Manufacturing

What is Active Pharmaceutical Ingredients?
Active Pharmaceutical Ingredients (APIs) are the bioactive molecules of any drug used in pharmaceuticals, which cause the desired therapeutic action in the body of a human being. APIs are the central core of all drugs, chemical or biological and are responsible for the efficacy, strength, and safety of the drug. APIs are synthesized by various complex chemical synthesis, fermentation, biotechnological, or extraction processes based on the type of drug. The ultimate product formulation of a drug is the combination of the API and excipients or inactive ingredients that facilitate drug delivery and stability.
Key Applications Across Industries:
APIs are generally categorized as synthetic chemical APIs and biological APIs (bio-APIs) of living origin, e.g., recombinant proteins, monoclonal antibodies, and vaccines. The API industry is an essential sector in the world healthcare landscape because it dictates the availability, affordability, and quality of pharmaceutical products. Major production centers are India, China, the USA, and selected areas of Europe, with India and China collectively representing a major portion of global API production due to cheaper costs and strong chemical manufacturing facilities. The industry is strongly regulated by agencies like the US FDA, the EMA, and WHO to maintain compliance with good manufacturing practices (GMP). Over the last few years, the market has experienced an increasing trend towards high-potency APIs (HPAPIs), biopharmaceutical ingredients, and contract manufacturing services (CDMOs/CMOs) based on rising demand for personalized medicine and outsourcing by pharmaceutical innovators.
What the Expert Says: Market Overview & Growth Drivers
The global active pharmaceutical ingredients market reached a value of USD 245.59 Billion in 2024. According to IMARC Group, the market is projected to reach USD 368.98 Billion by 2033, at a projected CAGR of 4.40% during 2025-2033. The global API market is growing tremendously owing to the expanding incidence of chronic diseases like cancer, diabetes, and cardiovascular diseases, which are treated with long-duration medicines and treatment regimens. The aging world population coupled with increased healthcare consciousness has further broadened the market for both generic and branded pharmaceuticals and consequently driven API consumption.
Moreover, the emergence of biologics and biosimilars has revolutionized the pharma industry, as the biotechnological breakthroughs paved the way for the synthesis of extremely specialized APIs for precision therapy. Expansion of the generic pharmaceutical industry, especially in developing countries, is another major impetus, driven by governments and healthcare systems seeking to contain the cost of treatment. This has created a demand for low-cost API production, forcing pharmaceutical producers to contract out manufacturing to Indian and Chinese API manufacturers, where economies of scale and advanced chemical synthesis expertise make them competitive. Furthermore, technological innovations including continuous manufacturing, green chemistry, and advanced purification techniques are improving production efficiency and quality control. The COVID-19 pandemic also underscored the strategic importance of APIs, leading many countries to focus on supply chain localization and diversification to reduce dependency on imports. Regulatory support and government incentives for domestic API manufacturing, such as India’s Production Linked Incentive (PLI) scheme, are accelerating global capacity expansion. The increasing trend towards high-potency APIs, applied in oncology and autoimmune therapies, and rising R&D expenditure on new drug molecules continues to fuel the API market's growth wave globally.
Case Study on Cost Model of Active Pharmaceutical Ingredients Manufacturing Plant:
Objective
One of our clients reached out to us to conduct a feasibility study for setting up a medium scale active pharmaceutical ingredients manufacturing plant.
IMARC Approach: Comprehensive Financial Feasibility
We developed a comprehensive financial model for the setup and operation of a proposed active pharmaceutical ingredients manufacturing plant in India. This plant is designed to produce 10,000 tons of active pharmaceutical ingredients (paracetamol / acetaminophen) annually.
Manufacturing Process: The production of Active Pharmaceutical Ingredients (APIs) entails a series of closely regulated and controlled procedures aimed at creating the active chemical or biological entity that causes a drug's therapeutic action. The process can generally be divided into two broad stages: synthesis (or production) and purification (or downstream processing). During the synthesis stage, APIs are manufactured either by chemical synthesis, fermentation, bioprocessing, or by extraction from natural sources, depending on the character of the molecule. For chemically manufactured APIs, various reaction steps are performed in reactors, wherein raw materials and intermediates are subjected to specific chemical conversions under controlled temperature, pressure, and pH conditions. In biologically originated APIs, microbial or mammalian cell cultures are employed to produce the target protein or compound, followed by cell harvesting. For naturally occurring APIs, plant or animal extracts are treated to isolate the target compound. Following synthesis, the product is purified by processes such as filtration, crystallization, centrifugation, and drying to eliminate impurities and by-products. Sophisticated purification techniques like chromatography and ultrafiltration are also usually applied to achieve high purity levels. The final solid or liquid API is then put through quality testing for attributes such as potency, stability, and particle size distribution. Lastly, the cleaned API is filled and stored under controlled environmental conditions prior to shipment for formulation as dosage forms like tablets, injections, or capsules. Throughout the process, there is tight compliance to Good Manufacturing Practices (GMP) and regulatory standards for assuring the safety, efficacy, and consistency of the product for pharmaceutical use.
Get a Tailored Feasibility Report for Your Project Request Sample
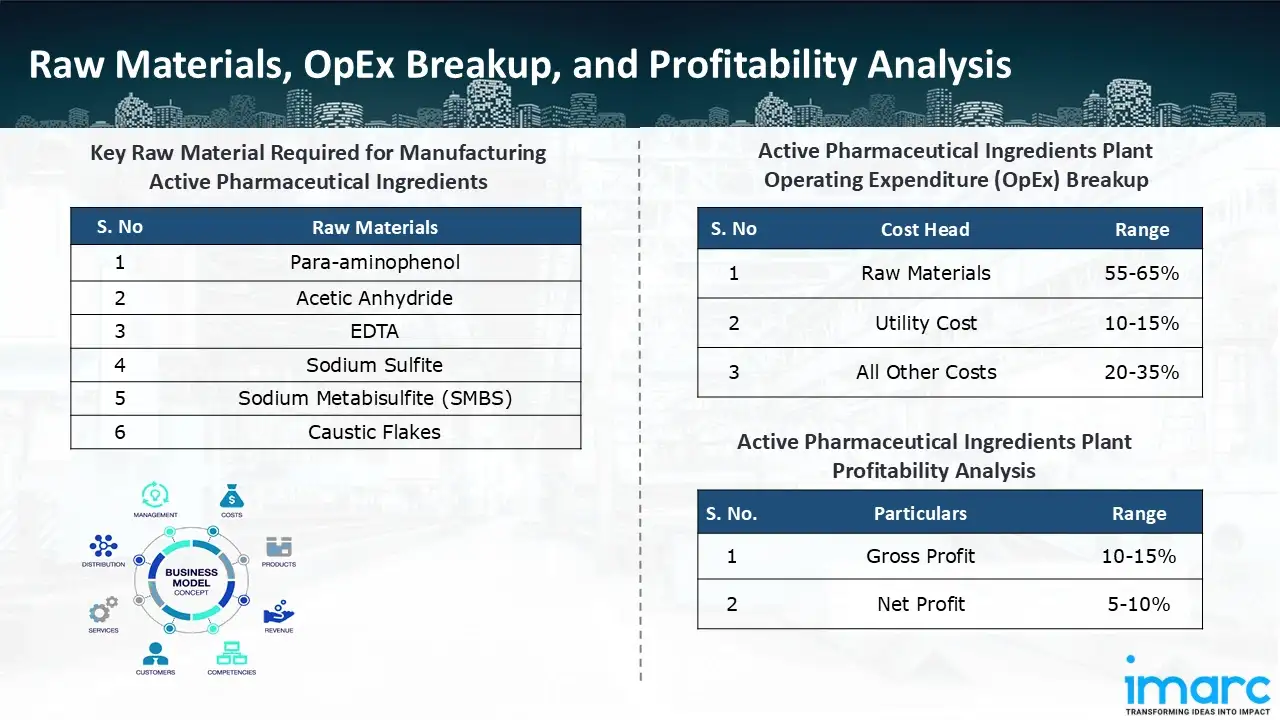
Raw Material Required:
The basic raw materials required for active pharmaceutical ingredients manufacturing include:
- Para-aminophenol
- Acetic Anhydride
- EDTA
- Sodium Sulfite
- Sodium Metabisulfite (SMBS)
- Caustic Flakes
Machineries Required:
- Storage Tanks for PAP And Acetic Anhydride
- Feeders / Weigh Hoppers
- Pumps for Liquid Handling
- Glass-Lined / Stainless-Steel Stirred Tank Reactor
- Neutralizer
- Cooling Crystallizer (Jacketed Tanks with Agitator)
- Neutch Filter
- Centrifuge
- Tray Dryer / Vacuum Dryer / Fluidized Bed Dryer
- Pulverizer
- Hammer Mill / Pin Mill / Jet Mill
- Vibrating Sieve
- HPLC
- UV Spectrometer
- Moisture Analyzer
- Melting Point Apparatus
- Air Compressor
- Storage Drums (Intermediate Bulk Containers)
- Controlled Environment Storage System
Techno-Commercial Parameter:
- Capital Expenditure (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. Opex in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth. Furthermore, raw material cost in an active pharmaceutical ingredients manufacturing plant ranges between 55-65%, utility cost ranges between 10% to 15%, and all other costs ranges between 20-35% in the proposed plant.
- Profitability Analysis Year on Year Basis: We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the projected period. Moreover, gross profit margins lie between a range of 10-15%, and net profit lie between the range of 5-10% during the income projection years, highlighting strong financial viability and profitability.
Conclusion & IMARC's Impact:
Our financial model for the active pharmaceutical ingredients manufacturing plant was meticulously developed to meet the client’s objectives, providing an in-depth analysis of production costs, including raw materials, manufacturing, capital expenditure, and operational expenses. By addressing the specific requirements of manufacturing 10,000 tons of active pharmaceutical (paracetamol / acetaminophen) ingredients annually, we successfully identified key cost drivers and projected profitability, considering market trends, inflation, and potential fluctuations in raw material prices. This comprehensive financial model equipped the client with valuable insights into strategic decision-making, demonstrating our commitment to delivering high-quality, client-focused solutions that ensure the long-term success of large-scale manufacturing ventures.
Latest News and Developments:
- In May 2025, the U.S. Food and Drug Administration (USFDA) issued a Form 483 with two observations after inspecting Dr. Reddy's Laboratories' API (Active Pharmaceutical Ingredients) production facility in Middleburgh, New York, the company reported on Saturday.
- In April 2025, the U.S. Food and Drug Administration (USFDA) has classified Medispray Laboratories, Cipla's wholly owned subsidiary, as a voluntary action indicated (VAI) facility for its API production plant in Goa.
- In July 2024, Pfizer Inc. declared that its highly automated Active Pharmaceutical Ingredient (API) manufacturing facility in Singapore's Tuas Biomedical Park has been extended. The 429,000-square-foot, state-of-the-art facility addition, which will cost SGD$1 billion, will manufacture a variety of small molecule APIs for Pfizer's antibiotic, pain, and oncology medications and supply them to markets worldwide. It has started commercial product manufacturing after passing all the final performance qualification exams.
Why Choose IMARC?
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104