Paracetamol Manufacturing Cost Analysis: Affordable Relief, Engineered Costs

What is Paracetamol?
Paracetamol, also referred to as acetaminophen, is an analgesic and antipyretic compound that is commonly used worldwide for the treatment of mild to moderate pain and the management of fever. The mechanism of action occurs mainly in the central nervous system, where it inhibits the mechanisms that regulate pain and body temperature, but lacks anti-inflammatory properties. Paracetamol is appreciated both for its safety profile when taken according to recommended dosages and for its usability in various patient categories, starting from pediatric patients, the elderly, right down to patients with antacids that cannot tolerate non-steroidal anti-inflammatory compounds. Paracetamol is produced as an active pharmaceutical ingredient with various forms of dosage formulations.
Key Applications Across Industries:
Paracetamol is very popular in health facilities globally for its effectiveness, affordability, and vast acceptance among patients. The major indication for the use of paracetamol includes the treatment of mild to moderate headaches, toothaches, muscle aches, back pain, joint pain, and post-operative pain. In addition, paracetamol can be found in health facilities for the control of fever caused by infection, immunization, and inflammatory diseases, thus acting as a common antipyretic drug.
In pediatric practice, paracetamol is today one of the most widely used drugs in the management of fever and pain in children, being marketed in syrups, suspensions, drops, and chewable tablets. It has been widely preferred over other pain relievers, considering its safety in the gastrointestinal tract and the least effect on platelet function. In adult practice, paracetamol has been widely used in clinical practice as a primary analgesic, especially in patients who are sensitive to the cardiovascular, renal, or gastrointestinal systems.
Paracetamol is also utilized in combined preparations along with other pharmacologically active substances, including caffeine, antihistamines, and decongestants, for relieving cold, influenza, and sinus symptoms. In a healthcare facility, intravenous paracetamol is applied for immediate pain and fever relief, particularly if oral administration is not possible. Besides, paracetamol is utilized within chronic pain management programs as part of combined pharmacologic therapies. Such flexibility of paracetamol applications ensures that it finds extensive applications in medicine.
What the Expert Says: Market Overview & Growth Drivers
The global paracetamol market reached a value of USD 778.18 Million in 2024. According to IMARC Group, the market is projected to reach USD 1,136.68 Million by 2033, at a projected CAGR of 4.09% during 2025-2033. The paracetamol market across the globe is supported by a number of factors related to the accessibility and acceptability of the drug, the common trend of prevalent diseases, the capability of the drug-manufacturing industries, and the acceptance by the drug-regulating authorities. The major factor supporting the growth of the market across the globe will, however, be the consistently high prevalence of the common pain and fever-related illnesses experienced by all age groups.
The fact that Paracetamol is included in the list of essential medicines in many countries is an important factor that promotes its usage by many public healthcare systems, hospitals, and humanitarian organizations around the globe. The affordability of this medicine and its long-established safety profile make it accessible in many markets, whether it is developed or emerging. The growing population and an aging population, together with an increase in healthcare usage, further strengthen the demand, especially among elderly people suffering from chronic pain.
Another major contributing factor is the rise in generic manufacturing facilities for pharmaceuticals. The fact that paracetamol has gone off-patent makes it possible for several companies to develop generics of the drug, which helps in mass production. Improvements in pharmaceutical manufacturing and formulation help in increased production.
Rising awareness about drug safety is also a factor that has added to the market growth of paracetamol. Since paracetamol is safer than some other available analgesics, it is prescribed to patients with some specific contraindications, which has added to its importance. Rise in the trend for self-medication and over-the-counter drugs has also positively added to the demand for paracetamol.
Public health preparedness and stockpiling for the treatment of infectious diseases is another contributing factor. The combined effects of strong medical acceptance, the essential medicine category, demographics, the scalability of the manufacture of generics, and the practice of self-medication are responsible for the sustained widespread use of paracetamol.
Case Study on Cost Model of Paracetamol Manufacturing Plant:
Objective
One of our clients reached out to us to conduct a feasibility study for setting up a medium scale paracetamol manufacturing plant.
IMARC Approach: Comprehensive Financial Feasibility
We developed a comprehensive financial model for the setup and operation of a proposed paracetamol manufacturing plant in India. This plant is designed to manufacture 300 tons of paracetamol annually.
Manufacturing Process: The manufacturing process for paracetamol consists of a series of carefully controlled chemical synthesis, purification, and finishing steps aimed at yielding a high-purity active pharmaceutical ingredient (API) suitable for medicinal purposes. The process begins with the synthesis of the key intermediate p-aminophenol from phenol or nitrobenzene by nitration, reduction, and rearrangement reactions, which are carefully controlled. After the synthesis and purification of p-aminophenol, acetylation follows as a result of a reaction with acetic anhydride or acetyl chloride. The result is the transformation of the amine group into an amide part, yielding paracetamol as the main product. After the synthesis process has been completed, the reaction mixture undergoes controlled cooling and crystallization techniques to precipitate the paracetamol in the solid state. The crude precipitate is then filtered and separated and subjected to the washing process to eliminate the reactants and impurities. The process of purification is highly important since the final grade of the paracetamol has to have specific purity and particle size. This process may involve the use of re-crystallization. The purified paracetamol is dried using vacuum dryers and fluidized bed dryers to reach consistent levels. Milling and sieving are also performed. The chemical is also tested for quality during its production to determine whether it meets standards concerning chemical identity, strength, and impurities. The paracetamol is also tested to meet pharmacopeial standards. The final API product is packaged in a controlled environment before being distributed to formulation plants for use in preparing final products. The final product is required to meet standards concerning chemical identity, strength, and impurities.
Get a Tailored Feasibility Report for Your Project Request Sample
Raw Material Required:
The basic raw materials required for paracetamol manufacturing include:
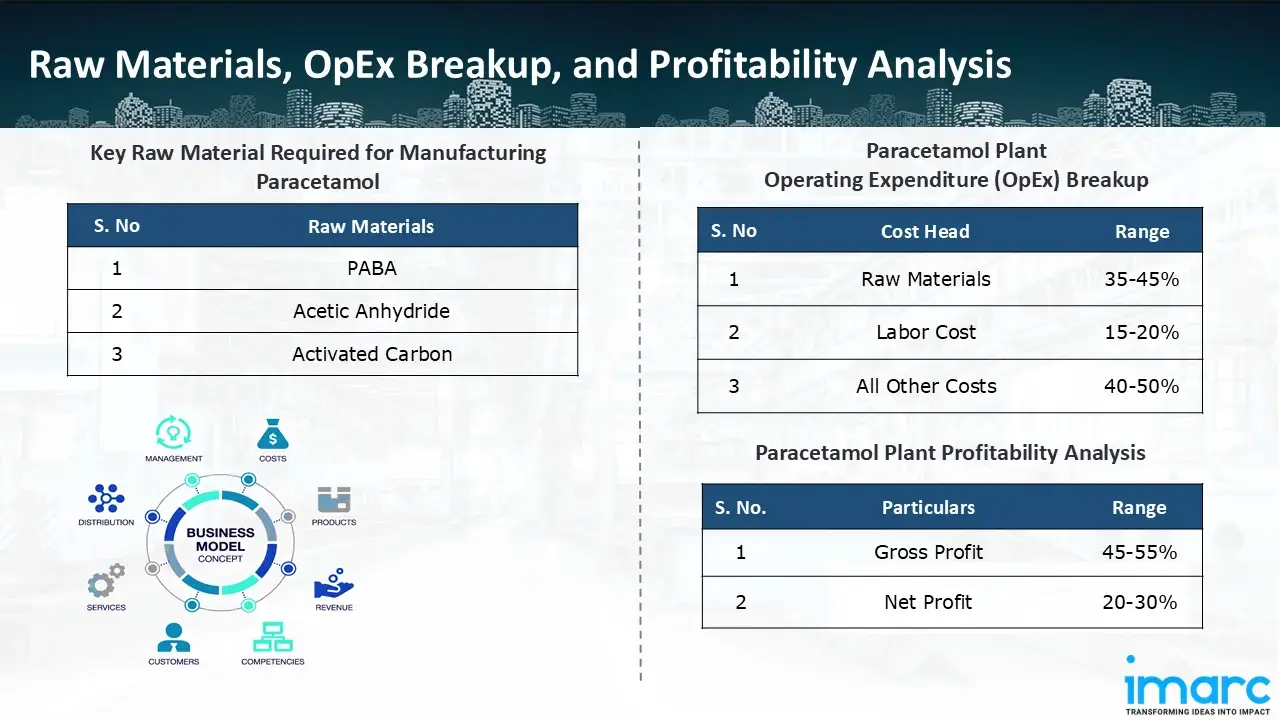
- PABA
- Acetic Anhydride
- Activated Carbon
Machine Section or Lines Required:
- Reactors
- Crystallization
- Drying
- Purification
- Tablet Press
Techno-Commercial Parameter:
- Capital Expenditure (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. OpEx in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth. Furthermore, raw material cost in paracetamol manufacturing plant ranges between 35-45%, labor cost ranges between 15% to 20%, and all other costs ranges between 40-50% in the proposed plant.
- Profitability Analysis Year on Year Basis: We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the projected period. Moreover, gross profit margins lie between a range of 45-55%, and net profit lie between the range of 20-30% during the income projection years, highlighting strong financial viability and profitability.
Conclusion & IMARC's Impact:
Our financial model for the paracetamol manufacturing plant was meticulously developed to meet the client’s objectives, providing an in-depth analysis of production costs, including raw materials, manufacturing, capital expenditure, and operational expenses. By addressing the specific requirements of manufacturing 300 tons of paracetamol annually, we successfully identified key cost drivers and projected profitability, considering market trends, inflation, and potential fluctuations in raw material prices. This comprehensive financial model equipped the client with valuable insights into strategic decision-making, demonstrating our commitment to delivering high-quality, client-focused solutions that ensure the long-term success of large-scale manufacturing ventures.
Latest News and Developments:
- In November 2025, Farmson Basic Drugs opened its cutting-edge Unit-VI production plant in Nandesari, Vadodara, Gujarat. This contemporary development represents a significant turning point in Farmson's transformation into a fully integrated paracetamol manufacturer. The INR 300 crore investment makes Unit-VI an essential component of Farmson's strategy for vertical integration.
- In January 2025, India’s Council of Scientific & Industrial Research (CSIR) announced developed indigenous technology to produce paracetamol, a widely used pain reliever and fever reducer. This innovation aims to make India self-reliant in paracetamol manufacturing, reducing dependence on imported ingredients.
Why Choose IMARC?
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104











