Pharmaceutical Manufacturing Cost Analysis: Prescription for Profitability

What is Pharmaceutical?
Pharmaceuticals are medical products, substances, or formulations involved in the development, manufacture, and regulation for the prevention, diagnosis, treatment, and management of diseases in humans as well as animals. They consist of active pharmaceutical ingredients (APIs) as well as finished products, which may include tablets, capsules, injectables, syrups, and topicals. Pharmaceuticals are produced under strict standards related to their quality, safety, and efficacy, ensuring tangible pharmacological responses. The pharmaceutical industry brings together scientific research, chemical synthesis, biotechnology, and sophisticated production methods to produce drugs that interact with biological responses in a controlled manner.
Key Applications Across Industries:
Pharmaceuticals form the backbone of modern healthcare systems because they cater to a vast range of biomedical needs pertaining to preventive, curative, as well as palliative components of healthcare. The principal use of pharmaceuticals lies within the treatment and management of infectious and chronic diseases such as infections, cardiovascular diseases, diabetes, respiratory diseases, neurological disorders, and cancers. Prescription pharmaceuticals are utilized within hospital, clinic, and outdoor settings for managing complex diseases and facilitating surgical processes to enhance patient survival and quality of life.
Pharmaceuticals are also employed in the arena of preventive health care. Vaccines, prophylactic drugs, and vitamin or mineral supplements can all prevent diseases and boost the immune system. Over the counter (OTC) medications can also promote self-care as an easily accessible source for treating common health conditions such as pain, fever, allergies, and gastrointestinal disorders.
In specialized medical fields, Pharmaceuticals aid in anesthetic procedures, critical care medicine, organ transplantation, and reproductive medicine. Biopharmaceuticals like Monoclonal Antibodies, Hormonal Therapies, and Gene Therapies aid in treating complex medical problems that were hitherto untreatable. Animal Pharmaceuticals aid in sustaining animal health and boosting farm productivity by controlling zoonoses.
Pharmaceuticals are also important components of the health programs of the Population. Moreover, they are deployed in disaster response programs as well as humanitarian health programs. More importantly, pharmaceuticals play supportive roles in medical research, in the diagnosis of diseases, as well as in monitoring.
What the Expert Says: Market Overview & Growth Drivers
The global pharmaceutical market reached a value of USD 1,645.75 Billion in 2024. According to IMARC Group, the market is projected to reach USD 2,820.43 Billion by 2033, at a projected CAGR of 6.2% during 2025-2033. The pharmaceutical market is influenced by trends in demography, disease prevalence, scientific developments, and increased provisions in the area of healthcare. Some of the most important forces influencing pharmaceuticals are the rising prevalence of chronic and lifestyle-related diseases like cardiovascular diseases, diabetes mellitus, cancers, and diseases associated with the immune system declining as a result of aging in populations worldwide.
Advancements in the field of medical sciences and pharmaceuticals have a substantial impact on market growth. Breakthroughs in biotech, genomics, and personalized medicine help bring targeted therapies and biologics into reality. Such advancements result in new areas of therapies and new therapeutic areas opening up. Investments in R & D also help in product differentiation in this market.
Improvements in healthcare infrastructure and accessibility of medications in emerging countries are also significant factors driving this market. Initiatives taken by governments to enhance healthcare infrastructure, insurance coverage, and support for essential medications are also contributing to increased consumption of medications globally. Manufacture of generic medications is an essential part of enhancing accessibility of these medications at an affordable cost to carry out large-scale treatments.
Growing health awareness and preventive healthcare practices also accelerate market growth. A higher emphasis on early diagnosis, immunization, and disease prevention encourages pharmaceutical use beyond curative purposes. Support for R&D, fast-track approvals for strategic therapies, and incentives for domestic manufacturing are also market growth contributors.
Moreover, preparedness in global health issues, pandemic preparedness capacities, and a resilient supply chain have also emerged as key areas that continue to emphasize the importance of pharmaceutical production in terms of strategy. Overall, trends in epidemiology, innovation in technology, healthcare expansion, and government support offset a slowdown in global pharmaceutical industry expansion.
Case Study on Cost Model of Pharmaceutical Manufacturing Plant:
Objective
One of our clients reached out to us to conduct a feasibility study for setting up a medium scale pharmaceutical manufacturing plant.
IMARC Approach: Comprehensive Financial Feasibility
We developed a comprehensive financial model for the setup and operation of a proposed pharmaceutical manufacturing plant in India. This plant is designed to manufacture 80 million tablets of pharmaceutical annually.
Manufacturing Process: The pharmaceutical manufacturing process is a series of operations that require strict control in order to ensure the safety and efficacy of the final product. The pharmaceutical manufacturing process begins with the procurement and qualification of raw materials such as active pharmaceutical ingredients (APIs) and excipients, whereby the raw materials are tested to conform to their purity and comply with the requirements of the pharmacopeial standards. For the manufacturing of APIs, there is synthesis or biotechnology in the form of fermentation or cell culture. manufacturing of the final dosage form involves accurate weighing and dispensing of the API or excipients, depending on the formulation. The API is mixed in such a way that it is well distributed. The formulation may undergo a process of granulation, which could be wet or dry, depending on the formulation of the drug, to make it easier to compress into tablets or fill into capsules in the case of solid oral dosages, or dissolve or suspend in solvents in the case of liquids, or aseptically fill in the case of injectables. Based on this formulation, further product processing operations like coating, polishing, or sterilization may be carried out depending on the nature of the product being manufactured. Packaging operations follow as the product is packaged into primary and secondary packaging materials with a view to protecting stability and facilitating traceability. During this process of manufacturing a product, various control operations like quality assurance tests during the manufacturing process ensure that all critical parameters for the product are met concerning content uniformity, dissolution, sterility, and stability. These operations occur under Good Manufacturing Practices (GMP).
Get a Tailored Feasibility Report for Your Project Request Sample
Raw Material Required:
The basic raw materials required for pharmaceutical manufacturing include:
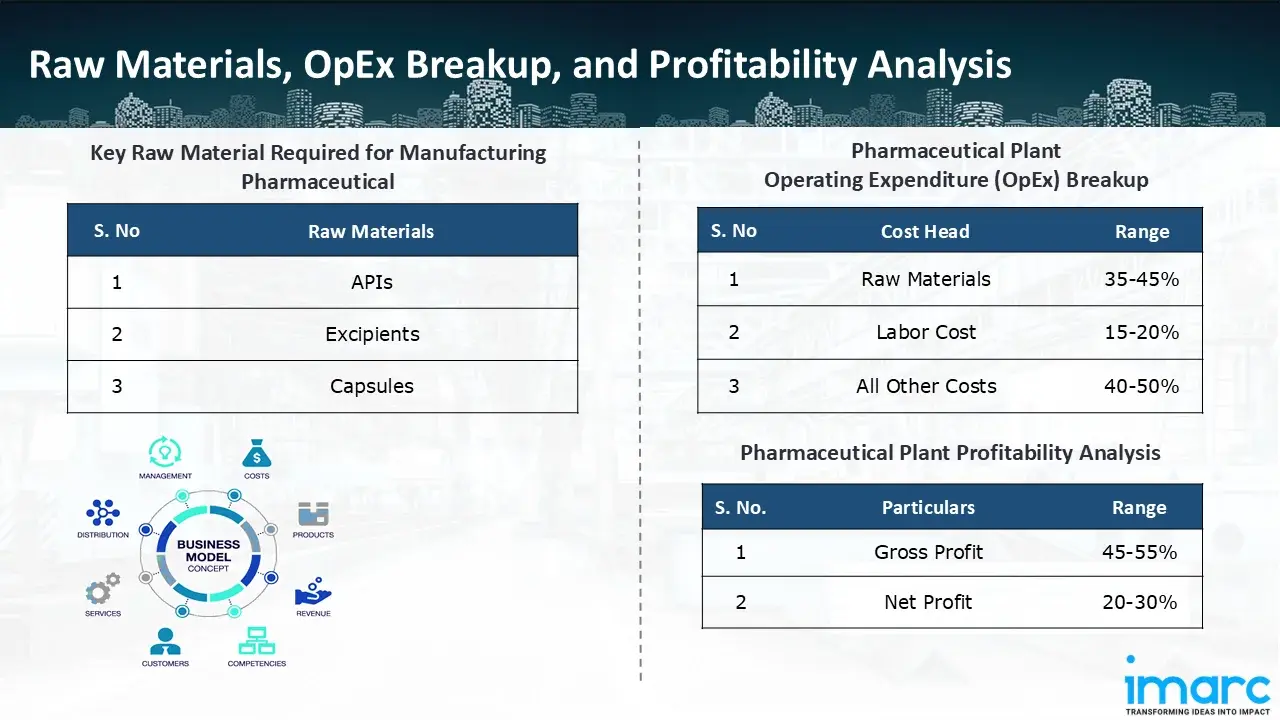
- APIs
- Excipients
- Capsules
Machine Section or Lines Required:
- Granulation
- Compression
- Coating
- Capsule Filling
- Packaging
Techno-Commercial Parameter:
- Capital Expenditure (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. OpEx in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth. Furthermore, raw material cost in pharmaceutical manufacturing plant ranges between 35-45%, labor cost ranges between 15% to 20%, and all other costs ranges between 40-50% in the proposed plant.
- Profitability Analysis Year on Year Basis: We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the projected period. Moreover, gross profit margins lie between a range of 45-55%, and net profit lie between the range of 20-30% during the income projection years, highlighting strong financial viability and profitability.
Conclusion & IMARC's Impact:
Our financial model for the pharmaceutical manufacturing plant was meticulously developed to meet the client’s objectives, providing an in-depth analysis of production costs, including raw materials, manufacturing, capital expenditure, and operational expenses. By addressing the specific requirements of manufacturing 80 million tablets of pharmaceutical annually, we successfully identified key cost drivers and projected profitability, considering market trends, inflation, and potential fluctuations in raw material prices. This comprehensive financial model equipped the client with valuable insights into strategic decision-making, demonstrating our commitment to delivering high-quality, client-focused solutions that ensure the long-term success of large-scale manufacturing ventures.
Latest News and Developments:
- In December 2025, Samsung Biologics announced that it has achieved a final deal to purchase Human Genome Sciences, a biopharmaceutical manufacturing company from GSK, for $280 million. This would be Samsung Biologics' first planned manufacturing presence in the United States.
- In September 2025, Eli Lilly and Company announced plans to construct a $5 billion manufacturing facility in Goochland County, just west of Richmond, Virginia. For Lilly's developing bioconjugate platform and monoclonal antibody portfolio, the new location will serve as the company's first fully integrated active pharmaceutical ingredient (API) and drug product facility.
- In June 2025, UCB, a multinational biopharmaceutical firm dedicated to enhancing the lives of those with serious illnesses, announced its plans to make a sizable investment in a brand-new, cutting-edge biologics manufacturing facility in the United States. The project is expected to serve UCB’s growing number of patients in the U.S., while delivering a total estimated economic impact of approximately USD 5 Billion.
Why Choose IMARC?
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104










