Rapid Diagnostic Test Kit Manufacturing Cost Analysis: Fast Results, Smart Economics

What is Rapid Diagnostic Test Kit?
A rapid diagnostic test (RDT) kit is a medical device designed to quickly detect specific biomarkers, antigens, antibodies, or nucleic acids linked to diseases—typically within minutes and without the need for sophisticated laboratory equipment. These kits often use lateral flow assays, immunochromatographic strips, or rapid molecular testing methods to deliver fast and reliable results. RDT kits are engineered for simplicity, portability, and accuracy, requiring minimal sample preparation and user training. Because they can be used outside of traditional lab settings, they are ideal for point-of-care testing in clinics, field operations, emergency settings, and even at home. The primary advantage of RDTs lies in their ability to provide immediate clinical insights, supporting early diagnosis, timely treatment, and effective disease control.
Key Applications Across Industries:
Rapid diagnostic test kits have become indispensable across healthcare, public health, veterinary medicine, and research due to their speed, accessibility, and versatility. In clinical settings, RDTs are widely used to detect infectious diseases such as malaria, dengue, influenza, COVID-19, HIV, hepatitis, and sexually transmitted infections. Their ability to deliver accurate results within minutes allows healthcare professionals to make quick decisions regarding treatment initiation, patient triage, and infection control, reducing the risk of further transmission.
Within public health and disease control programs, RDTs play a vital role in surveillance, outbreak management, and screening initiatives. They are commonly deployed in mass testing campaigns, border screening, refugee health programs, and emergency response operations, especially in low-resource or remote regions where laboratory facilities are limited. This decentralization of testing significantly improves access to diagnostics and strengthens health system responsiveness.
In blood banks and transfusion centers, rapid test kits are used to screen donated blood for infectious diseases, ensuring transfusion safety. Similarly, in veterinary healthcare, RDTs aid in the early diagnosis of infections in livestock and companion animals, supporting both animal welfare and the prevention of zoonotic diseases.
The growing market for home and self-testing kits is another major application area. Individuals can now monitor health conditions such as pregnancy, glucose levels, or infectious diseases privately and conveniently, empowering proactive health management. In research and epidemiology, RDT kits are invaluable for field data collection, on-site disease mapping, and rapid screening in resource-limited environments. Their combination of portability, affordability, and reliability underpins their expanding use across human and animal health sectors worldwide.
What the Expert Says: Market Overview & Growth Drivers
The global rapid medical diagnostic kits market reached a value of USD 19.42 Billion in 2024. According to IMARC Group, the market is projected to reach USD 31.12 Billion by 2033, at a projected CAGR of 5.4% during 2025-2033. The global rapid diagnostic test kit market is expanding rapidly, driven by rising healthcare demands for timely, cost-effective, and decentralized diagnostic solutions. One of the primary drivers is the increasing global disease burden, encompassing both infectious and chronic illnesses. Early and accurate diagnosis is essential for effective treatment and containment, and RDTs provide results far faster than conventional laboratory tests—improving patient outcomes and reducing disease spread.
Public health preparedness and epidemic response have become central growth factors. Governments and health organizations worldwide are prioritizing rapid testing infrastructure to enhance disease surveillance and outbreak response capabilities. The scalability of RDTs allows for large-scale deployment during emergencies, enabling quicker decision-making in the field.
Healthcare accessibility also fuels adoption. In regions with limited laboratory capacity, RDTs offer reliable, on-site testing that minimizes delays and dependence on centralized facilities. Their simplicity reduces the need for specialized equipment or personnel, making them ideal for rural and underserved communities.
Technological advancements continue to broaden the market. Modern RDTs exhibit improved sensitivity, specificity, and stability, increasing their reliability across different conditions. Innovations such as multiplex testing (detecting multiple pathogens in one assay), enhanced sample collection systems, and digital integration for result tracking and telemedicine support are further transforming the industry.
At the same time, consumer-driven trends including the growing popularity of home-based and self-administered diagnostic kits, reflect a shift toward personalized, preventive healthcare. Regulatory bodies have also supported market growth by developing clearer standards and approval pathways, ensuring product quality and public trust.
In summary, the global RDT kit market is being propelled by a convergence of factors: rising disease incidence, public health needs, healthcare system constraints, technological innovation, and consumer demand for convenient testing solutions. Together, these dynamics are driving sustained investment and innovation in rapid diagnostic test kit manufacturing worldwide.
Case Study on Cost Model of Rapid Diagnostic Test Kit Manufacturing Plant:
Objective
One of our clients reached out to us to conduct a feasibility study for setting up a medium scale rapid diagnostic test kit manufacturing plant.
IMARC Approach: Comprehensive Financial Feasibility
We developed a comprehensive financial model for the setup and operation of a proposed rapid diagnostic test kit manufacturing plant in India. This plant is designed to manufacture 20 million of rapid diagnostic test kit annually.
Manufacturing Process: The manufacturing of rapid diagnostic test (RDT) kits involves a highly controlled sequence of biochemical, mechanical, and assembly operations, each designed to ensure consistent accuracy, reliability, and compliance with international regulatory standards. Production begins with assay development and raw material preparation, where biological reagents such as antibodies, antigens, enzymes, or nucleic acid probes are produced, purified, and characterized for quality and activity. These reagents are then formulated into buffers and conjugates under tightly controlled environmental conditions to maintain stability, reactivity, and long-term performance.
For lateral flow–based RDTs, manufacturing centers on the preparation of membrane-based test strips. Nitrocellulose or other specialized membranes are selected as the primary test substrate. Using automated striping equipment, capture reagents are precisely dispensed to form the test and control lines, ensuring reproducibility and alignment. Separately, the conjugate pads, which contain reactive particles such as gold nanoparticles or latex beads coated with antibodies or antigens are prepared and treated to ensure optimal release when the sample is applied. The sample pad and absorbent pad are also treated to control sample flow rate, filtration, and capillary action, ensuring uniform test performance.
Once individual components are ready, they are assembled into continuous laminated sheets following a defined sequence: sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad. The laminated sheets are then cut into narrow strips using precision cutting systems. Depending on the final product format, these strips are either housed within plastic cassettes or configured as dipstick tests.
In the case of molecular or cartridge-based RDTs, additional operations are required, including reagent loading, sealing, and microfluidic assembly using automated dispensing, filling, and sealing equipment. These systems are designed to handle sensitive reagents under sterile or humidity-controlled environments to maintain product integrity.
Throughout manufacturing, rigorous quality control (QC) is performed at multiple stages. Tests verify sensitivity, specificity, flow rate, signal intensity, and reproducibility. Only batches that meet performance criteria proceed to final assembly and packaging. The completed test strips or cartridges are then packaged with accessories such as buffer solutions, sample droppers, and instructions for use under controlled temperature and humidity conditions. Final packaging and labeling are conducted in compliance with regulatory frameworks such as ISO 13485, GMP, and relevant regional standards (e.g., FDA, CE, or WHO prequalification). Each batch is fully traceable, with documentation ensuring sterility (where applicable), stability, and consistent product performance throughout its shelf life.
Modern RDT kit manufacturing emphasizes automation, precision dispensing, and stringent quality management, ensuring that every test delivered to healthcare providers or consumers performs accurately, safely, and reliably supporting rapid, point-of-care diagnostics worldwide.
Get a Tailored Feasibility Report for Your Project Request Sample
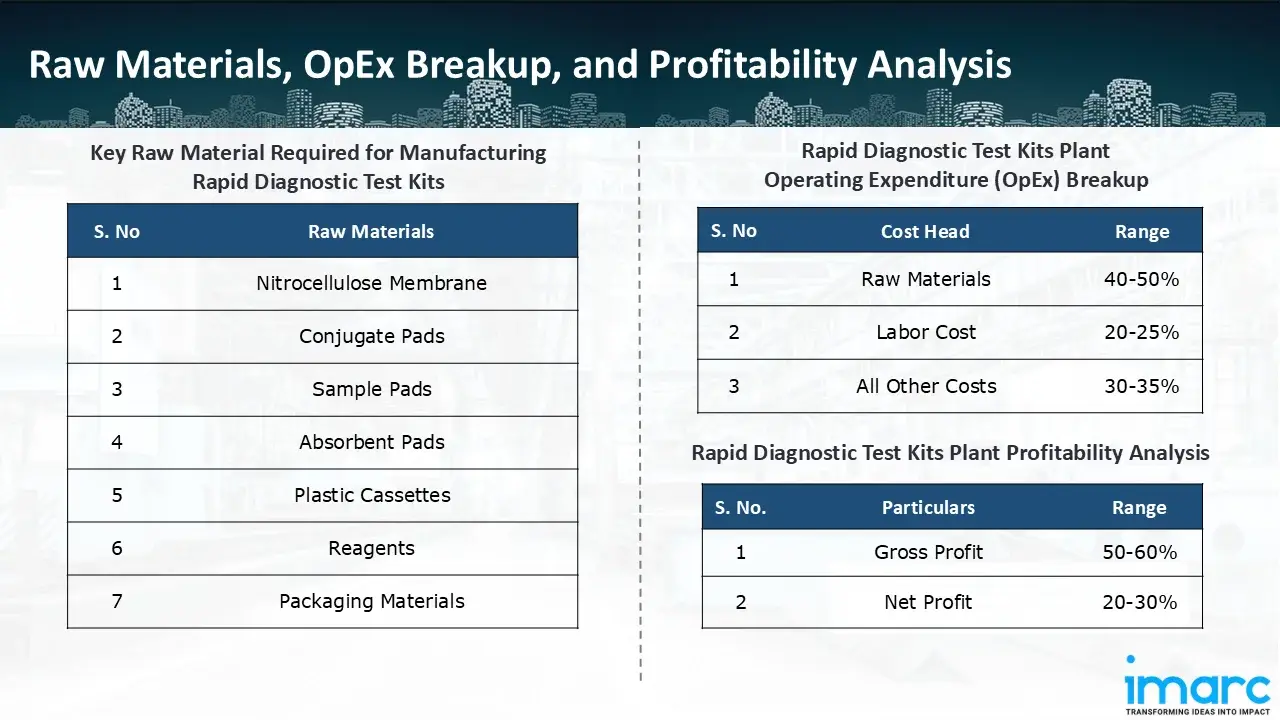
Raw Material Required:
The basic raw materials required for rapid diagnostic test kit manufacturing include:
- Nitrocellulose Membrane
- Conjugate Pads
- Sample Pads
- Absorbent Pads
- Plastic Cassettes
- Reagents
- Packaging Materials
Machine Section or Lines Required:
- Reagent Preparation
- Membrane Coating
- Drying
- Cutting
- Assembly
- Packaging
- Quality Control
Techno-Commercial Parameter:
- Capital Expenditure (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. OpEx in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth. Furthermore, raw material cost in rapid diagnostic test kit manufacturing plant ranges between 40-50%, labor cost ranges between 20% to 25%, and all other costs ranges between 30-35% in the proposed plant.
- Profitability Analysis Year on Year Basis: We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the projected period. Moreover, gross profit margins lie between a range of 50-60%, and net profit lie between the range of 20-30% during the income projection years, highlighting strong financial viability and profitability.
Conclusion & IMARC's Impact:
Our financial model for the rapid diagnostic test kit manufacturing plant was meticulously developed to meet the client’s objectives, providing an in-depth analysis of production costs, including raw materials, manufacturing, capital expenditure, and operational expenses. By addressing the specific requirements of manufacturing 20 million units of rapid diagnostic test kit annually, we successfully identified key cost drivers and projected profitability, considering market trends, inflation, and potential fluctuations in raw material prices. This comprehensive financial model equipped the client with valuable insights into strategic decision-making, demonstrating our commitment to delivering high-quality, client-focused solutions that ensure the long-term success of large-scale manufacturing ventures.
Latest News and Developments:
- In October 2025, the U.S. Food and Drug Administration (FDA) has given its Flowflex® Plus RSV + Flu A/B + COVID Home Test 510(k) certification, according to a recent announcement by ACON Laboratories, Inc., a prominent worldwide manufacturer of medical devices. The Flowflex Plus RSV + Flu A/B + COVID Home Test (K251749) is an over the counter (OTC) fast antigen test that may be given to customers in the privacy and comfort of their own homes. This new test will be produced domestically in ACON's cutting-edge plant in San Diego, California.
- In June 2025, Nigerian firm Codix Bio Ltd. intends to produce millions of HIV and malaria test kits for the local and regional market at its new plant outside Lagos in order to fill the void left by cuts at the U.S. donor organisation USAID.
- In January 2025, the World Health Organisation (WHO) has prequalified SD BIOSENSOR's STANDARD G6PD Test. This milestone represents a breakthrough in international efforts to eradicate Plasmodium vivax (P. vivax) malaria and enhance malaria treatment.
Why Choose IMARC?
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104










